Driving Innovation Using AI & Imaging
If artificial intelligence (AI) and clinical trials have been on your mind, this is a must-watch session.
Andrea Falkoff, Senior Director, Product Management at Medidata moderated a panel discussion at Medidata NEXT New York 2023 with thought leaders from RadMD, BIOVIA, and AstraZeneca, discussing innovation in AI—the much-debated technology. The group shared insights into AI’s place in medical imaging and its impact, adoption, possibilities, and challenges. Read on to learn more.
Transforming Clinical Trial Imaging
Imagine the workflow for clinical trial imaging, step-by-step, and the time taken to complete individual manual tasks. Multiply that by one million—that’s how many imaging uploads Medidata Rave Imaging has processed. Now consider that over 50% of clinical trials use medical imaging, and 95% of all oncology trials use it. AI is the key to effectively processing and managing such high volumes and levels of complexity.
Andrea walked the audience through a standard imaging workflow, showing how AI-assisted image analysis (Figure 1) makes a difference right now and how further advancements will transform the process in the near future.
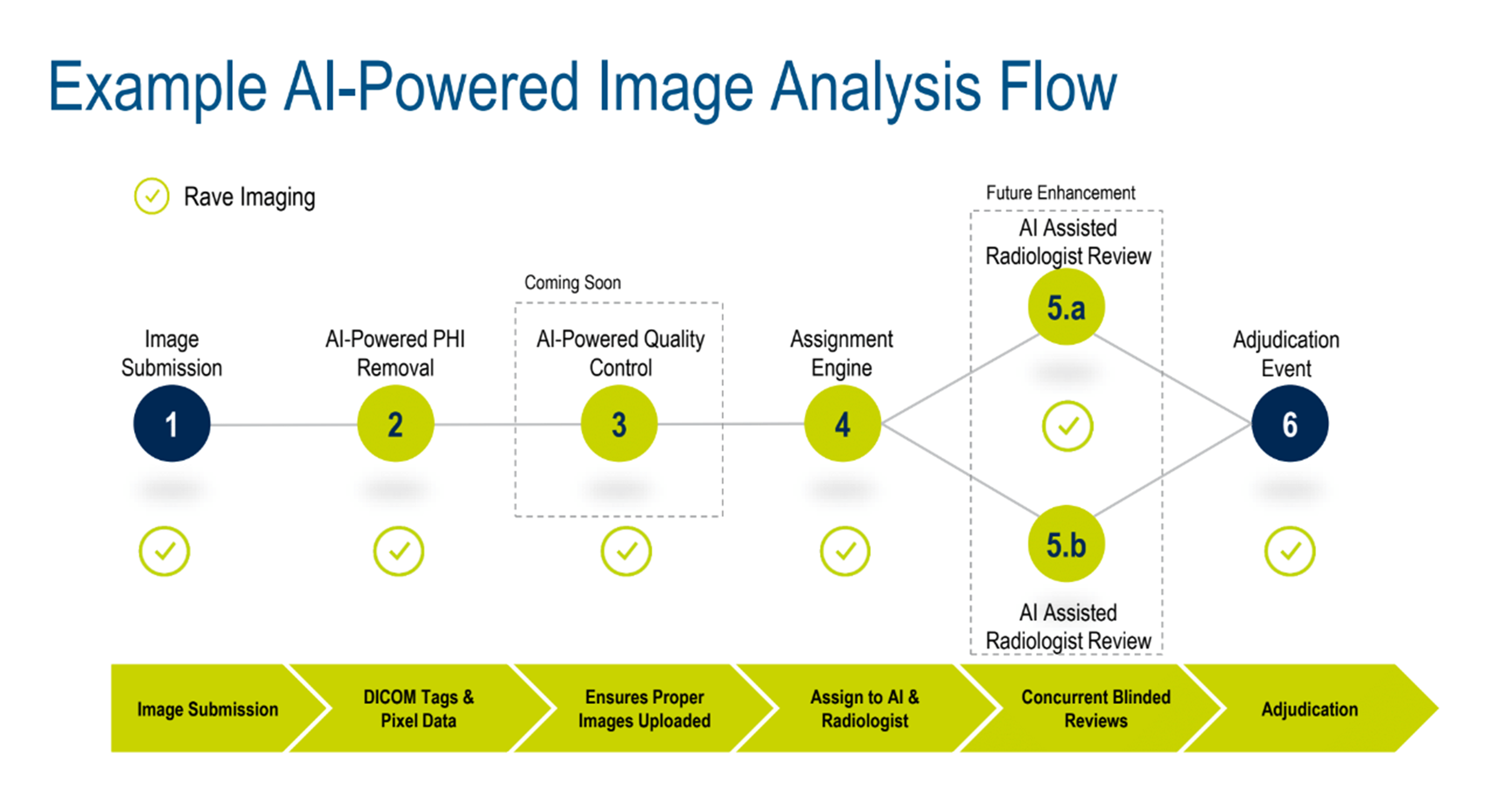
Figure 1. Example of an AI-powered image analysis flow.
BIOVIA’s Pipeline Pilot* was used to show how AI brings together pipeline data science, pipelining, and advanced analytics using a three-step process: read and process data, (clinical images and video from multiple sources and formats), build and apply machine learning (ML) models, and implement and operationalize (automated QA, diagnostics, and reporting).
Together, this process curates, cleans, and prepares data for modeling, then runs protocols to perform the necessary assessment. Live data is used to analyze multiple models and create dynamic dashboards.
Its prediction capabilities—a true differentiator and benefit—are being integrated into Medidata’s Rave Imaging soon, providing AI-powered analytics that can create bespoke AI algorithms that run on every image uploaded to the Medidata platform.
Improving Imaging Quality Control with AI
AI-assisted quality control (QC) is a key part of image processing before going to the readers. An oncology image QC (Figure 2) use case was used to highlight challenges addressed by AI-assisted solutions. Examples included human decisions and errors, delays, variances in international regulations, and image quality. Additionally, sponsors’ expectations are that reads will be delivered faster, but manual processes aren’t scalable and can lead to delays and errors. AI would efficiently fill gaps in knowledge and automate processes.
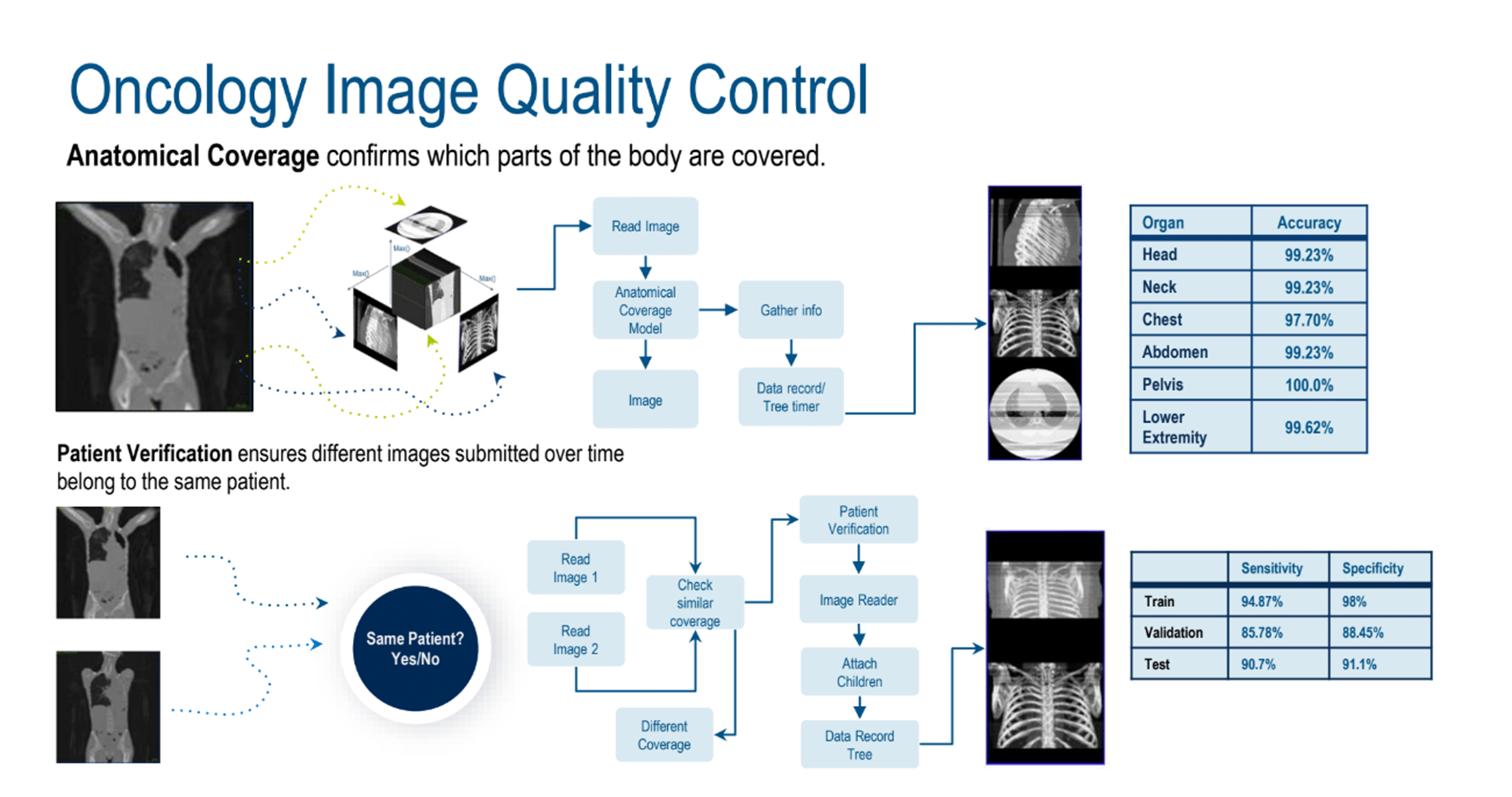
Figure 2. Oncology image quality control.
Making the Most of AI-Machine Learning (ML) Models
With AI-assisted benefits in mind, the many potential uses in other areas would include video analytics, adaptive clinical trials, drug lead optimization, formulation optimization, and automation, to name a few. (Figure 3)
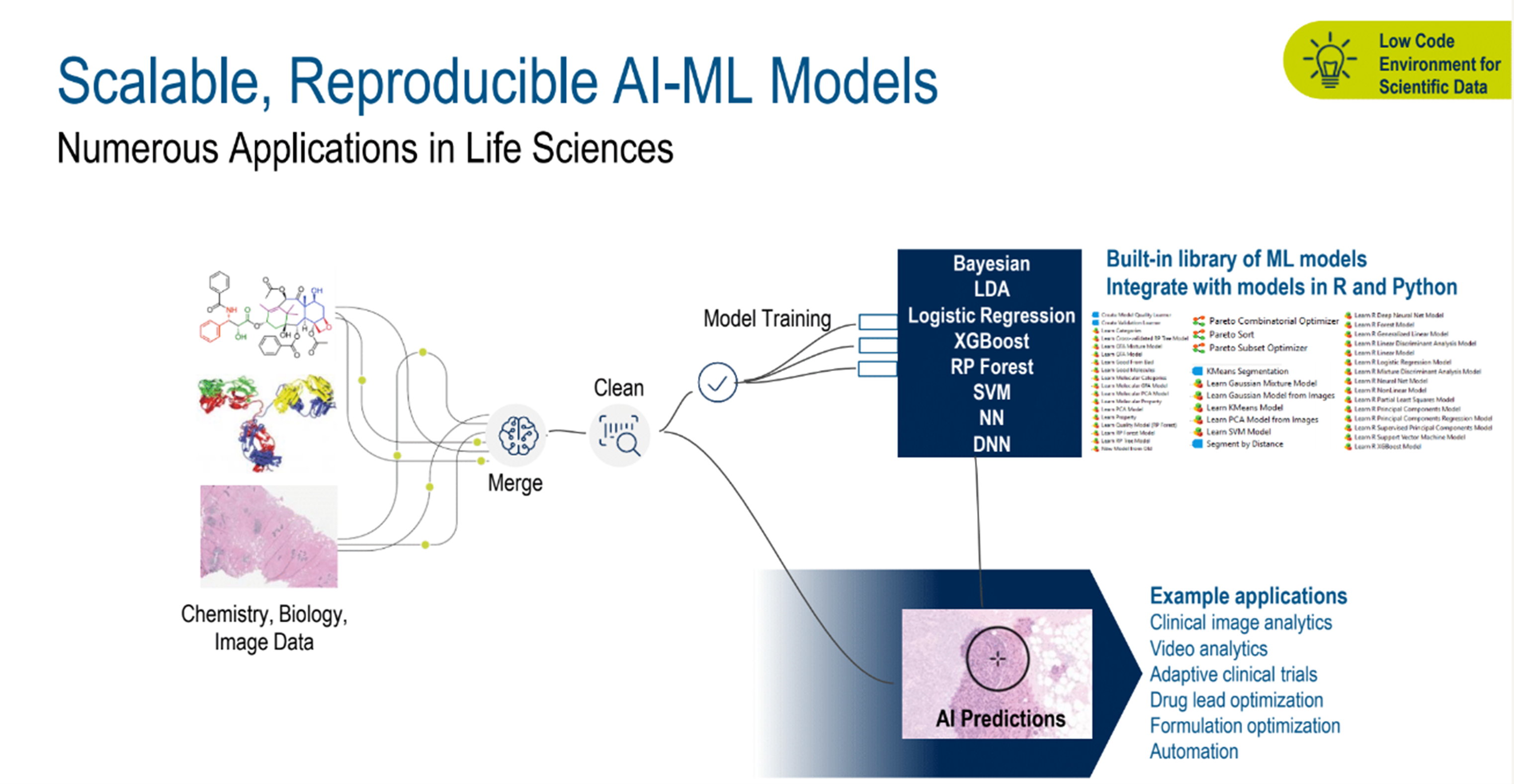
Figure 3. Scalable, reproducible AI-ML models.
Exploring this, a proof of concept example for clinical video analysis (Figure 4) showed how imaging can be taken to another level. Three challenges were addressed: Video (generates data that requires human judgment and expert analysis); QC (cost reduction is an ongoing sponsor goal) Data (sponsors are looking to get more information from video data).
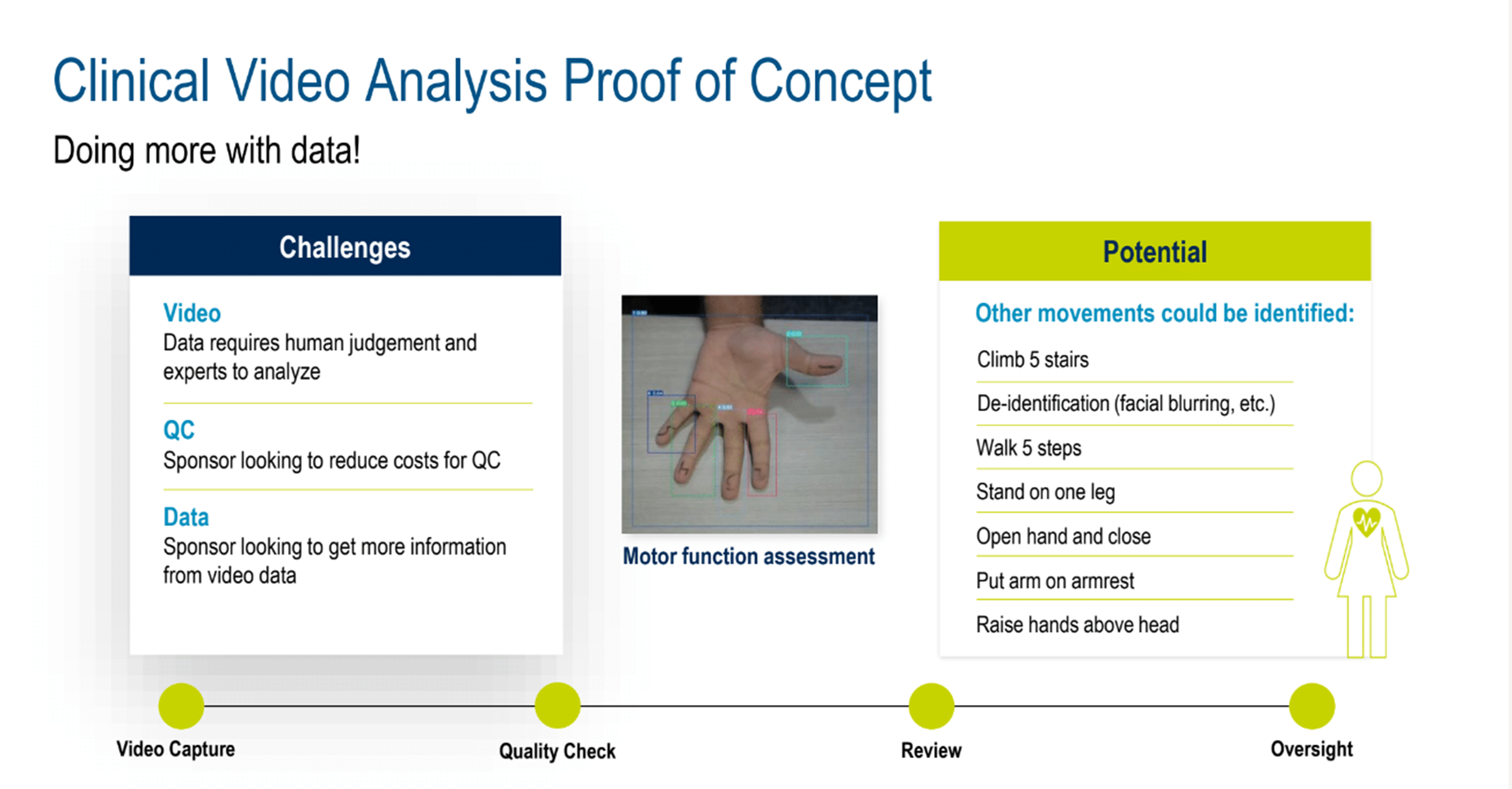
Figure 4. Clinical video analysis proof of concept.
AI-assisted video analysis can identify important physical movements, adding invaluable data for studies. A lively discussion ensued, highlighting examples of AI’s ability to capture nuanced movements that would normally need human review, such as precise hand movements, change in gait, or macro motions, that aid clinical analysis, comparison, and assessment.
Next, the topic of better data management was discussed. Can the various layers of siloed data from multiple studies be used more efficiently? Absolutely. Medidata can remove many inefficiencies and challenges by centralizing data in a master imaging library—another innovation the Medidata Imaging team will launch towards the end of 2024. (Figure 5).
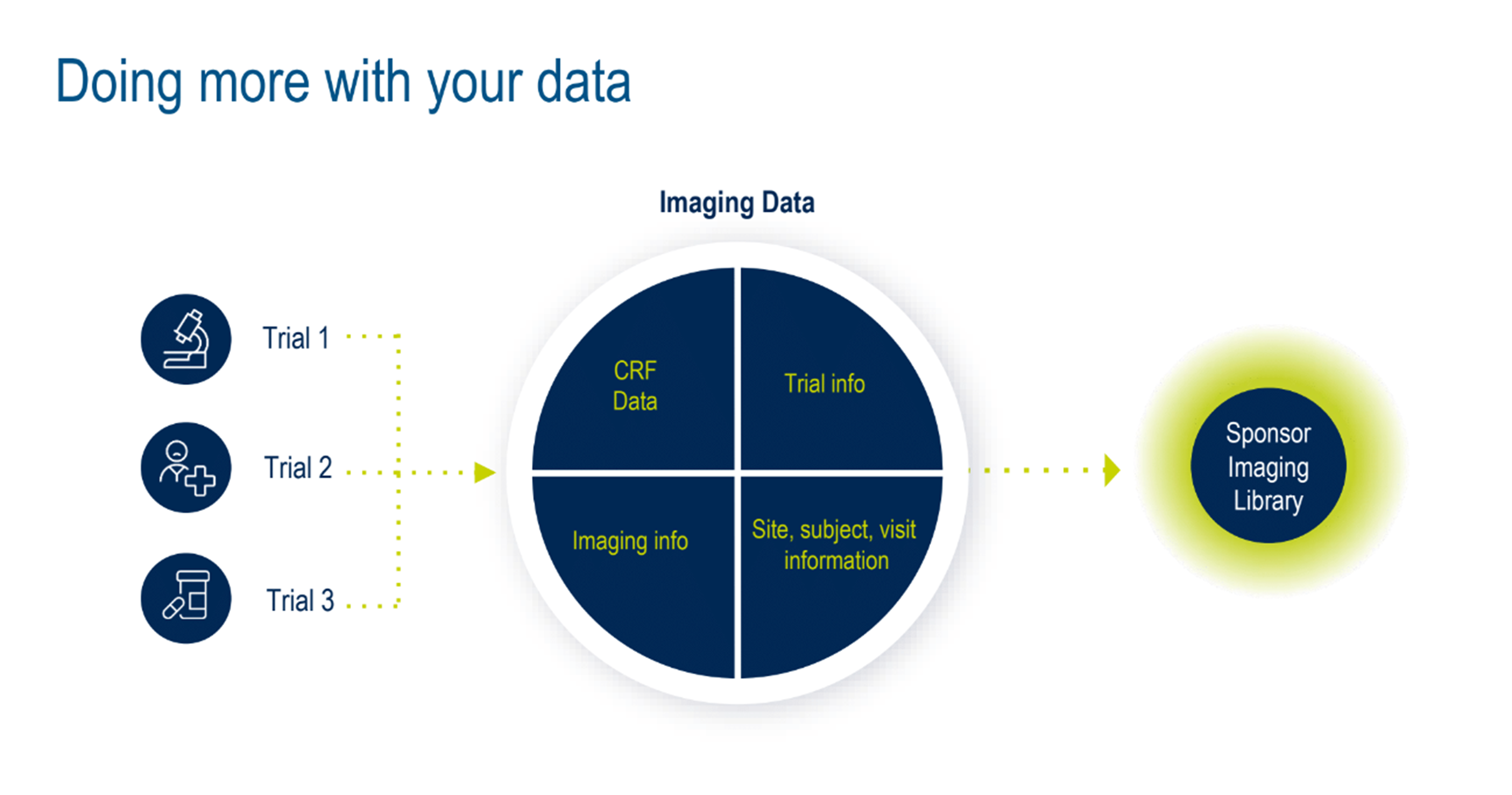
Figure 5. Doing more with your data.
Will AI Replace All Manual Clinical Trial Imaging Tasks?
Put simply, no. The panelists stated that while AI is a powerful tool, it could not replace all tasks. Dr. Gutierrez stated that it will help radiology to evolve in the clinical setting, but not with blinded readings due to deep levels of complexity stemming from the variety of endpoints from trial to trial. However, it will be a useful tool for central radiologists to improve quality and reduce read times. Agreement on this was universal from the panel.
In Conclusion – The Pathway to Industry Adoption
There was great enthusiasm and optimism for medical imaging and AI’s transformational capabilities, including mention of early adopters who are leading the charge. The session concluded with a thought-provoking discussion on challenges and the pathway to AI adoption. Mr. Vilardi felt that acceptance is needed from industry leadership and regulatory bodies to see much wider adoption. He and an audience member suggested that an industry consortium should build a body of proof to achieve this.
We agree that industry leaders should insist on proof for any solution, especially when it comes to AI. After all, not all AI is created equal. But from a sponsor’s perspective, as an early adopter, this does not mean a blind leap of faith if they choose the right solution and partner.
Medidata Rave Imaging has supported 34,000+ global sites, 272,000+ patients, 1M+ imaging exams uploaded, 75+ core labs/imaging CROs connected, and 1080+ Imaging Trials. It has streamlined processes and timelines, with an average of 6-8 weeks to build studies, 20+ fewer hours spent reconciling per month, and an 86% reduction in data query rates.
BIOVIA is one of the industry’s innovators and pioneers. Over 20 years, its Pipeline Pilot has proven itself as an advanced data science, AI/machine learning, cheminformatics, and structure-based modeling solution.
Medidata Rave and BIOVIA Pipeline Pilot will be integrated later this year, taking the most powerful unified platform in the industry to another level.
Watch the full session here to learn more about the technology transforming clinical research.
*BIOVIA and Medidata are part of the Dassault Systèmes Group
Contact Us

