Clinical Data Studioのメリット

データレビューを加速化し、データの品質を保証
Clinical Data Studioにより、データマネージャーは、全ての臨床試験データを一箇所でレビューすることが可能になります。データマネージャーは、AIと自動化によるサポートを得て、レビューのサイクルタイムを1サイクル当たり最大80%短縮することができます。

リスクを管理し、いち早く課題を特定して対処
Clinical Data Studioでは、組み込まれたAIおよびスマート解析によって、クリニカルオペレーションチームがデータ品質上のリスクや患者の安全性上のリスクを一元的に監視・管理・低減することが可能となります。

患者の安全性に関わるシグナルをいち早く特定
すべてのデータが一箇所に集約されているため、治験依頼者、メディカルモニター及び治験実施施設は包括的な患者のナラティブプロファイルを用いて、ペイシェントジャーニー(発症から回復に至るまでの患者の体験)を簡単に設定し、可視化することが可能となります。
Clinical Data Studioでは、メディカルモニターはすべての集約された患者データにわたり、有効性と安全性の傾向を完全に把握することができます。これにより、予期しないパターンや新たに現れるパターンを特定し、潜在的な患者の安全性の問題を発見することができます。また、腫瘍学に特化した分析とダッシュボードにアクセスし、複雑なRECIST 1.1データを簡単に取得することができます。

技術上の複雑性および負荷を低減
Clinical Data Studioとは、Medidata Platform上で提供される体験であり、本ソリューションでは、システムの維持のために別のシステムの追加や統合を行うのではなく、Rave EDCのデータに直接アクセスします。わずか3日間でセットアップ・稼動開始が可能であり、ローコード/ノーコード環境であるため、運用に高額な管理サービスは不要です。それどころか、本ソリューションは、データの(他社のシステムからの)インポート、統合および標準化を簡略化することにより、テクニカルリソースへの依存を軽減します。
実証例
プログラミング作業時間の短縮
リストの作成にかかる時間を短縮
データレビューの自動化
データレビューのサイクルタイムを短縮
シンプルな設定
KRIの設定時間を短縮
セットアップ期間の短縮
わずか3日間でセットアップ・稼動
Clinical Data Studioの主な特長
Clinical Data Studioは、一元的な質の高いユーザー体験下で様々な機能を提供することにより、お客様のデータの品質にプラスの効果をもたらします。
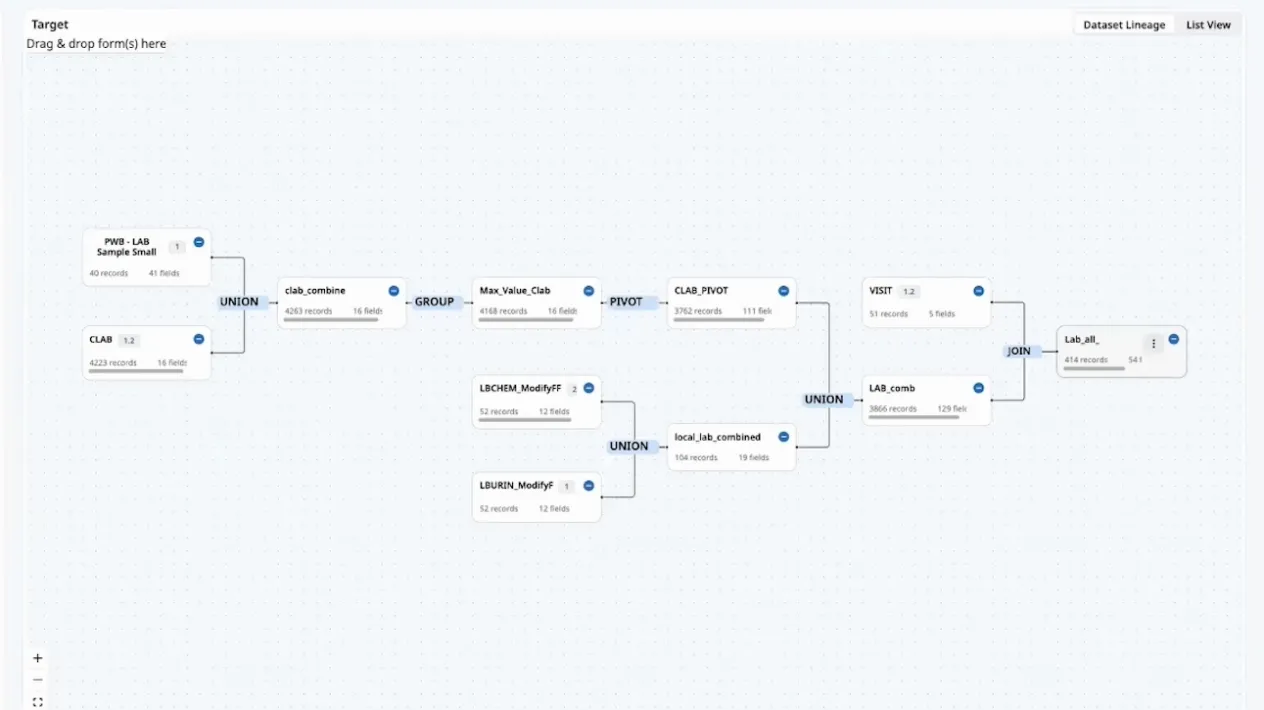
ノーコード/ローコードでのデータの統合・標準化・変換
Clinical Data Studioでは、ノーコード/ローコード環境で、セルフサービスでのデータのインポート、バリデーション、単位変換やカスタムデータセットの統合を行うことが可能となるため、複数のデータソース(メディデータのシステムおよび他社のシステム)のデータが集約・標準化され、これらのデータをデータマネージャー、セントラルモニタリング担当者やメディカルモニターがレビューや解析に利用することができるようになります。
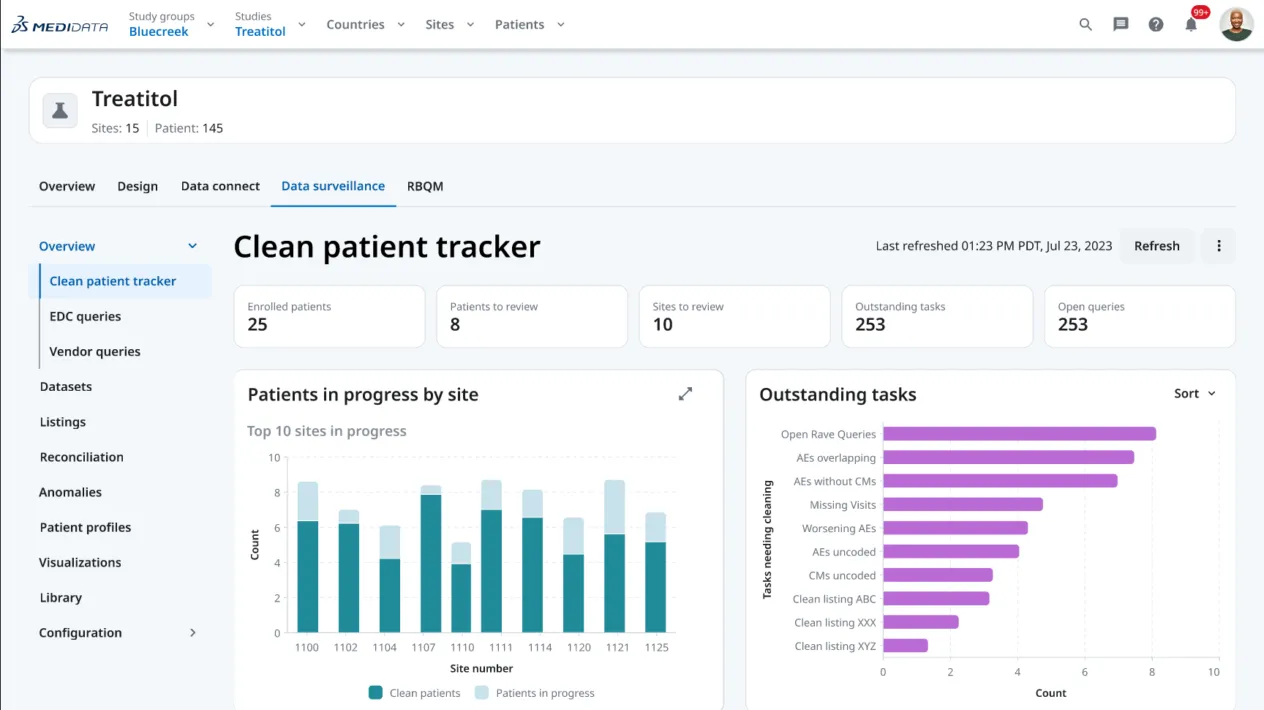
データのレビュー・監視
Clinical Data StudioのData Surveillance機能により、プログラミングなしでのデータレビューリストの作成、Rave EDCのクエリの自動生成、AIによるデータ照合のサポートが実現し、データの容易な可視化、完全な患者プロファイルの作成、データクリーニングの進捗状況の追跡が可能となります。
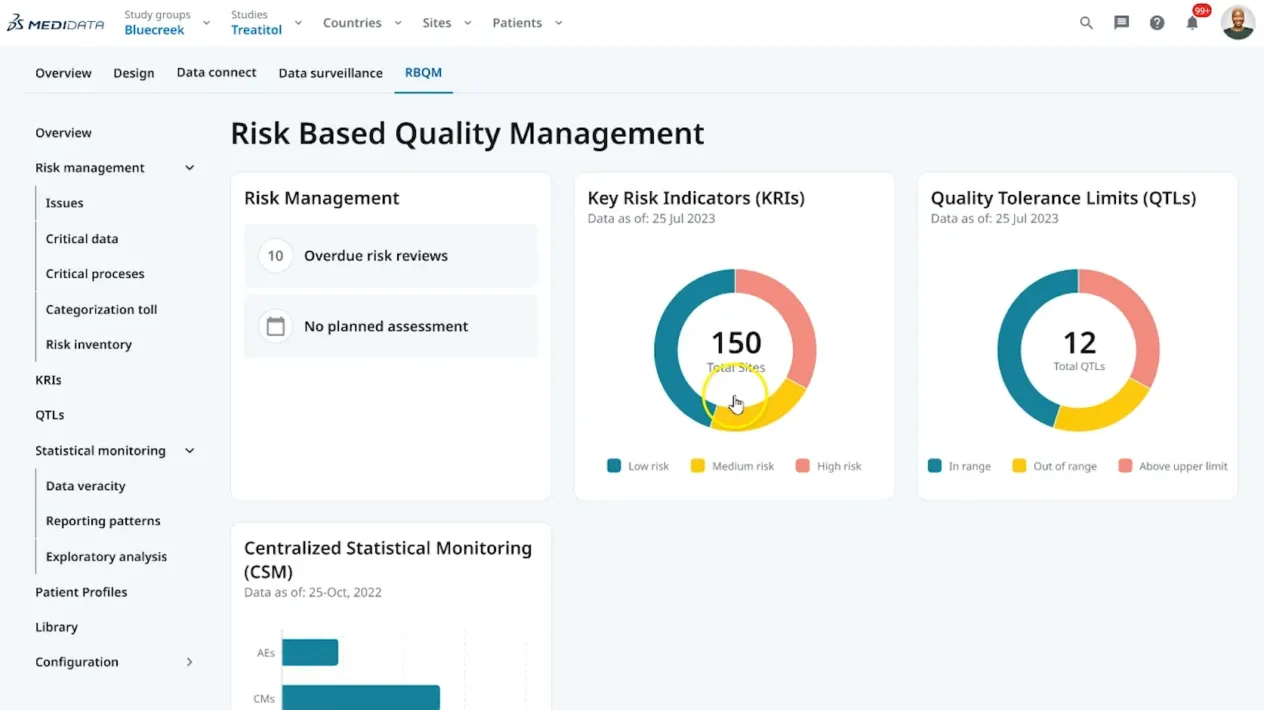
リスクベースの品質管理(RBQM)
Clinical Data Studioでは、一箇所で、全ての臨床データを対象として、リスクベースの品質管理戦略を実行することができます。KRI(重要リスク指標)やQTL(品質許容限界)を迅速に設定・監視することが可能です。AIを利用して、異常や通常と異なる傾向を特定することにより、治験実施施設のパフォーマンスやコンプライアンス上のリスクを明らかにします。データ品質や患者の安全性に関わる課題を、いち早く特定することができます。
お客様の声

「Clinical Data Studio(旧称Detect)によって、深く掘り下げた データ分析を行うことが可能となりました。実際に、本製品のお蔭でデータの全体像を見渡すことができるようになりました。」
–Heidi McIntyre(Moderna社中央データモニタリング部ディレクター)

「私たちは、安全性部門、メディカル、臨床部門、そして関係者全員を同一のプラットフォーム上に集約して、信頼できる唯一のデータソースを使用することを検討する必要があります。 Clinical Data Studio(旧称Detect)は、これを可能にしてくれます。」
– Haiyan Wei(臨床データサイエンス部シニアバイスプレジデント)
詳細を見る

データマネージャーのための革命/進化への道筋。臨床データの「5つのV」の全領域にわたり、データ品質を向上させるためのガイド
臨床試験のデータ管理は、過去10年間で急激に複雑性を増しています。
データマネージャーは、ますます多様化するデータソースから、膨大な量のデータを収集・処理・分析する作業において、前例のない難題に対処しています。
臨床試験データの革新的進化の先頭に立ち続けるために、テクノロジーを利用して、「5つのV」-量(Volume)、種類(Variety)、速度(Velocity)、正確性(Veracity)および価値(Value)による、臨床データ戦略・臨床データ管理プロセスに関する情報の取得と、その最適化を継続的に実施するための方法をご覧ください。
RBQM:データ品質のための結合組織
私たちの体が、全ての部位が互いに調和して連動した場合にのみ機能するのと同様に、RBQMは、試験における結合組織の役割を果たします。本電子ブックでは、人体から得た着想に基づき、臨床試験のデータ品質を向上させる7つの方法について検討します。

テクノロジーによる、クリニカルデータマネジメントからクリニカルデータサイエンスへの変革のサポート
本稿では、クリニカルデータマネジメントがクリニカルデータサイエンスへと進化し、今やデータマネージャーが全データおよび全データ品質の管理者となった進歩の概略について説明します。革新的戦略の重要な理念と最先端のテクノロジーに焦点を当てながら、先見的で効率が高く、多くの洞察が得られるデータ管理エコシステムを実現します。

臨床データ品質に関するブログ記事シリーズ
患者中心とデータ中心、データプラットフォームアーキテクチャが重要である理由、RBQMやAI等のトピックについて考察した、メディデータの臨床データ品質・テクノロジーの専門家によるブログ記事をご覧ください。

