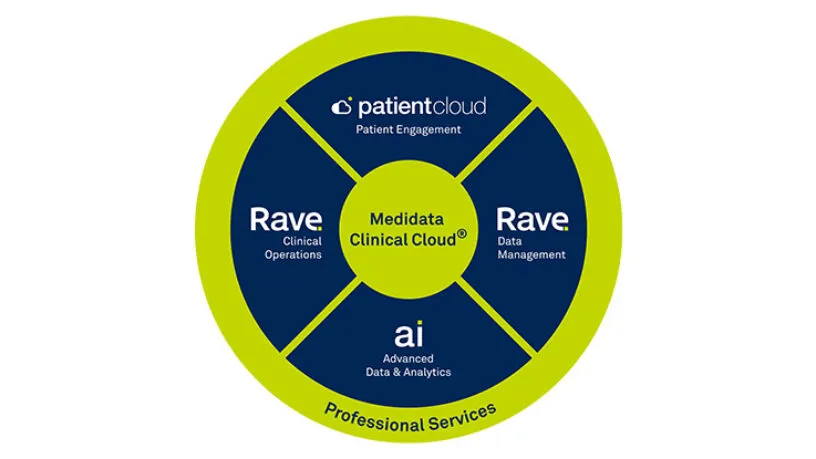
Medidata’s Site Insights Program
Sites are more than our end users, they are our partners.
The Medidata’s Site Insights Program was designed to partner with sites in new and novel ways.
We’re creating a new paradigm of transparency, efficiency, and site-centricity in clinical research to ensure the site voice is embedded in everything we do and that every action we take contributes to improving sites’ and patients’ lives.
Sites are more than our end users, they are our partners.
To be patient-centric, be site-centric.
Medidata is committed to solving the challenges preventing clinical research sites from providing a world-class patient experience and superior outcomes for our clients and CRO partners.
Eliminating Double Data Entry
For years, site personnel have manually re-entered data from other systems into their EDC software, while managing multiple logins. There’s so much back and forth, typing, and errors, resulting in more queries. Medidata’s Rave Companion significantly reduces data entry efforts for sites by providing a simpler and faster way to get source data from Electronic Health Record (EHR) and other systems into Rave EDC, all while having a single sign-on solution on the Medidata platform.

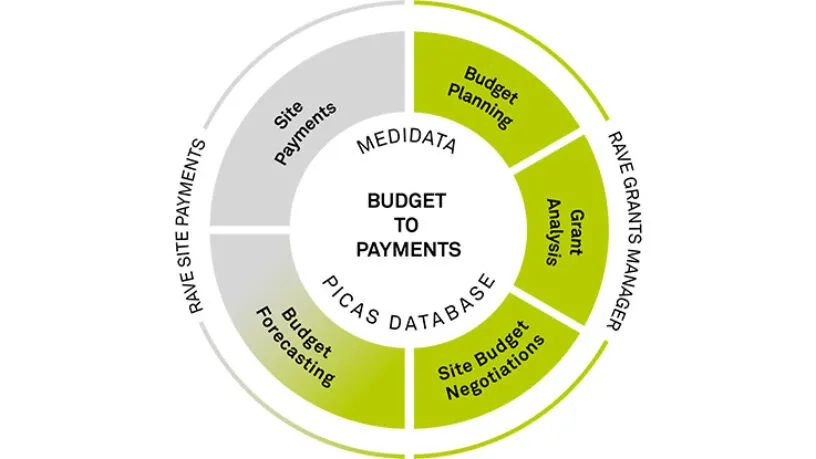
Paying Sites Right
Paying sites in a timely and accurate manner has been challenging for years. The cash flow of a site is a huge factor in the success of their participation and, ultimately, the success of the trial. Medidata Clinical Trial Financial Management experts dedicate their days to solving these issues through technology.

Fair Site Budgets
Getting paid by the Sponsor/CRO for all the work a site completes during a clinical trial depends a lot on a thorough budget negotiation.. Medidata’s budgeting solutions ensure trust among all stakeholders through objective, robust benchmarking data and full transparency in the process.
Site’s thoughts on DCTs
A site’s role in successfully executing Decentralized Clinical Trials (DCTs) is crucial. Sitesare at the frontline for patients and play many roles during a study: caretaker, educator, help desk, etc. Medidata has researched these activities since 2020 through several site surveys and is dedicated to keeping up with the feedback trends and adjusting our technology, accordingly.
Key Features
Our Site Advisory Board comprises executives from different types of sites and therapeutic expertise to harness the variety of perspectives needed to drive strategy and innovation. We are currently recruiting members and will launch this executive board in Q1 2025.
If you are a site executive and interested in joining our Site Advisory Board, complete the registration form. Click here
Our Site Tech Board powered by the Society for Clinical Research Sites (SCRS) hosts design studios with real site users and our product team to optimize current technology solutions and pilot innovations. Members have the opportunity to review new products and help optimize and innovate on behalf of all site users. Members meet 4-6 times a year (at least once in person), and being a member of SCRS is a prerequisite for our Site Tech Board at this time.
If you are interested in joining our Site Tech Board and are an active member of SCRS, complete the registration form. Click here
The Engage Site User Community is a web-based user community designed to streamline study agnostic information and provide quick access to critical information like site training and support. It’s an easy way to keep informed about Medidata, our products, upcoming events and respond to questionnaires and polls, providing site insights in real-time.
We bring the site voice with us to industry events and our NEXT series.
If you are a site staff member and are interested in serving on a panel or participating in various activities directly with other industry stakeholders, please let us know and we will be in contact with you as opportunities arise. Complete the registration form. Click here
It is critical for our teams to really understand how sites work. Medidata has built a network of sites, that host tours and are willing to educate Medidata team members about site operations, and allow them to better understand how Medidata products and services are used in the real world.
If you are from a site organization and would be willing to host a site visit for our Medidata team members to learn more about site operations and better understand how our products and services are used in the real world, please let us know and we will be in contact with you about next steps. Complete the registration form. Click here