Medidata Therapeutic Solutions
Medidata Therapeutic Solutions are designed to tackle the complexities of oncology and vaccine clinical trials safely and cost-effectively.
Medidata Therapeutic Solutions provides an integrated, cost-effective approach to supporting Phase 2 and Phase 3 oncology and vaccine studies. They help sponsors and CROs manage the rapid pace of research while focusing on regulatory compliance, patient safety, trial efficiency, and delivering life-saving treatments.

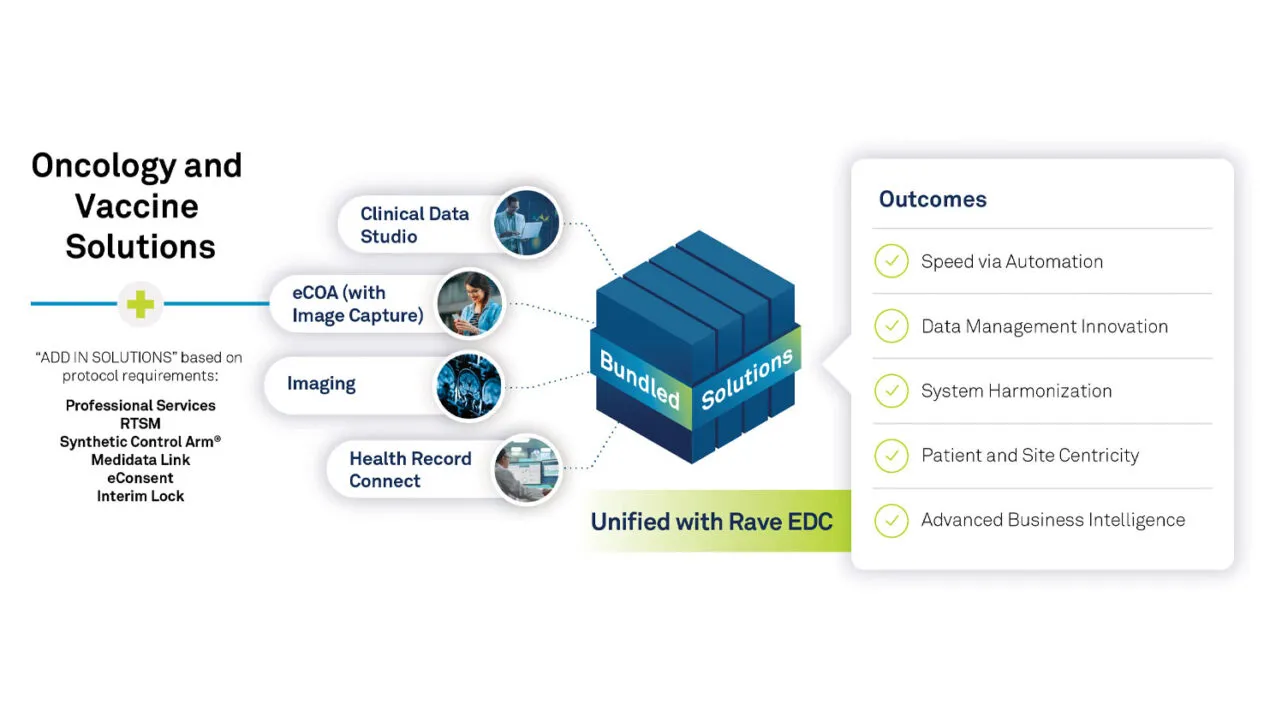
Four of Medidata’s powerful and innovative solutions – Clinical Data Studio, Medidata eCOA, Rave Imaging, and Health Record Connect – are bundled to drive oncology and vaccine trial quality and efficiency. Working with Rave EDC, these solutions provide the core of an efficient, cost-effective approach to accelerating your oncology and vaccine trials. Medidata Therapeutic Solutions offers a robust, integrated approach to managing your trials, with the added benefits of significant cost savings, scalability, and comprehensive support. This synergy simplifies the trial process and enhances the quality and reliability of trial outcomes.
In addition to the core solutions mentioned above, many other Medidata solutions, such as RTSM, eConsent, Medidata Link, Interim Lock, and Synthetic Control Arm, can be added to the bundle to cost-effectively and safely accelerate your trials.
Challenges
Sponsors and CROs face many challenges in rapidly and successfully executing oncology and vaccine trials, including trial design and curation, data complexity, patient recruitment, and the growing need for real-world data integration. The following table is a summary of these challenges by trial type:
Trial Design and Duration
Rare cancers, slow recruitment, adaptive protocols. Disparate technologies to support complexities.
Data Complexity and Monitoring
Complex data (genomics, biomarkers, imaging). Real-time monitoring of safety and efficacy.
Patient Recruitment and Retention
Small/scarce populations, slow recruitment, higher patient burden, and dropout rates.
Regulatory Requirements
High regulatory oversight, fast-track pathways.
Real-world Data Integration
Long-term efficacy, post-market surveillance, and diverse patient outcomes.
Trial Design and Duration
Large-scale, multi-country, rapid recruitment, especially in pandemics
Data Complexity and Monitoring
Focus on safety and immunogenicity with large populations, monitor rare events
Patient Recruitment and Retention
Recruit diverse healthy/at-risk populations quickly across regions
Regulatory Requirements
Comply with global regulatory standards, including emergency use during pandemics
Real-world Data Integration
Need to understand long-term vaccine effectiveness, monitor safety across diverse populations, and assess outcomes
Medidata Healthcare and Therapeutic Solutions Help Solve These Challenges
Experience
Medidata’s experience and expertise in oncology and vaccine trials are unparalleled and foundational to Medidata Therapeutic Solutions.
Experience
Years of oncology and vaccine experience
Trials
Oncology trials supported
Patients
Vaccine patients supported
Vaccines
COVID-19-related vaccine trials