Medidata Diversity Program
In order to deliver effective treatments broadly and inclusively, clinical trials must reflect diverse patient representation in all phases of the drug development process.
The Medidata Diversity Program:
- Builds diversity into every step of your clinical trial strategy
- Leverages data-driven site selection
- Engages patients in pre-and post-trial tools
- Provides insights from diverse patient advocates
- Trains the site network on Medidata’s DCT technology to achieve diversity throughout the clinical trial process.
The Importance of the Medidata Diversity Program

Build diversity into every step of your trial strategy
Set your study up for success by considering diversity before, during, and after your trial.
From site selection to protocol design, decentralization and patient engagement, the Medidata Diversity Program provides a suite of solutions that aims to cultivate diversity and ensure more equitable clinical trials.

Develop a trial strategy with trusted community partners
Actively infuse diverse patient voices into your protocol design to enhance trial experiences and strengthen patient trust.
Utilize patient-friendly technology solutions that have been reviewed and improved by trusted patient communities of diverse backgrounds.

Diversity success IS clinical trial success
Confidently create data and patient insights-driven diversity action plans for regulatory and operational success utilizing sites proven and ready to rapidly enroll diverse groups.
Cultivate diversity, close disparity
Enhance access to sites and information with patient-centric solutions.
Connect with sites that most effectively provide access to underserved communities while building groups of diverse, research-ready patients through pre-trial engagement and education.
A Multifaceted Approach to Diversity

Data-driven site selection
Confidently select high-performing sites using industry-wide, site-level data to identify sites that perform well operationally and have historically been successful in enrolling diverse patients to meet enrollment timelines and diversity enrollment targets.
- Understand diversity gaps and set realistic diversity goals for diversity action plans by benchmarking and baselining clinical trial diversity against the industry.
- Increase trial diversity by identifying sites that perform well operationally and can enroll a diverse population.
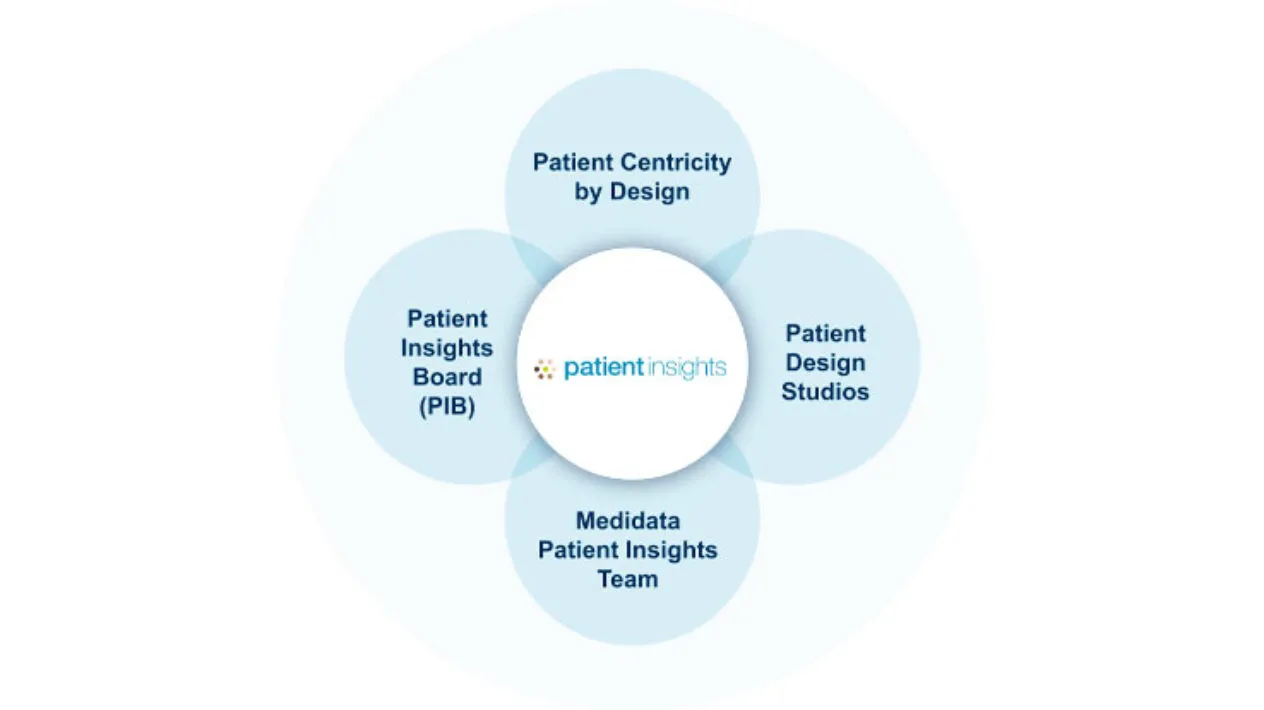
Insights from Patient Advocates
Create an experience designed for patients, by patients of diverse backgrounds.
- Test your protocol design and trial materials with diverse patient advocates and experts before bringing them to the wider audience.
- Design the most patient-friendly protocol to minimize burden and enhance the trial journey, enabling broader participation across diverse backgrounds.
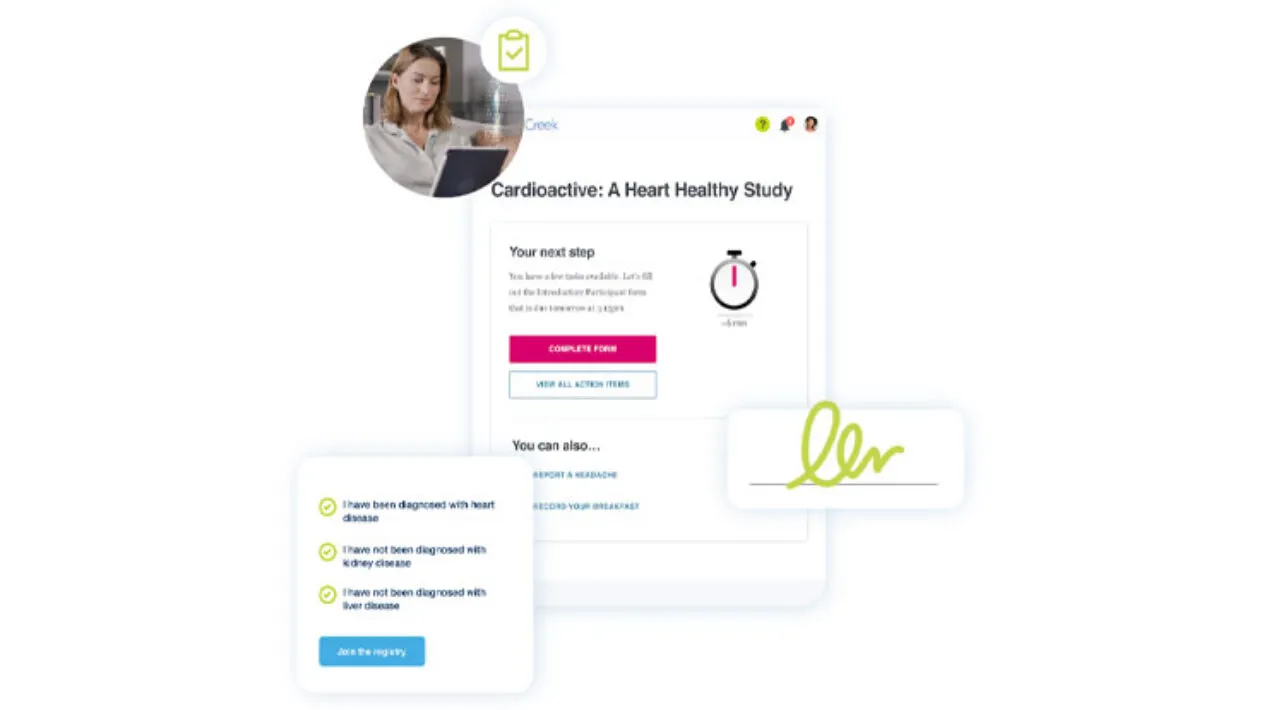
Pre-trial and post-trial engagement
Engage, educate, and empower patients from all walks of life, before the trial begins and after the trial ends.
- Educate a diverse pool of patients about clinical trial opportunities before the trial begins.
- Empower all patients with trial outcomes and future study opportunities after study completion, enabling patients to continue contributing to ongoing clinical trials.
Patient-Accessible Site Network
Access traditional, hybrid, and decentralized site network, all trained on Medidata’s DCT technologies, that is positioned and well-equipped to serve underrepresented populations.
- Increase clinical trial accessibility for diverse populations through Circuit Clinical’s site network.
- Equip local doctors to serve their community as a clinical research site.
- Add sites in communities where clinical research is not a care option.



