Autumn 2024 Newsletter

Guiding you through the challenges of the life sciences landscape
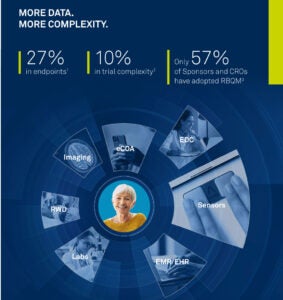
5 Strategies to Improve Clinical Data Quality
Clinical research professionals across all quality oversight functions face inefficient review processes. Data management and risk-based quality management (RBQM), clinical operations, and IT teams need access to the same error-free, low-latency data to achieve their goals - to shorten operational timelines, increase data quality, reduce risk, or improve patient safety.

November 13 & 14
Midtown Manhattan
Medidata NEXT returns to the Sheraton New York Times Square. And we’re bringing the unmistakable energy and innovation that makes NEXT so special. The biggest life sciences names, the most pressing topics—all tied together by immersive clinical trial experiences.
Join over 700 peers from clinical development and operations to data science and management at the industry’s premier event. Learn from top pharma and biotech leaders. Participate in more than 55 engaging sessions across two days. Get an immersive look at the latest innovations in clinical trials and patient engagement.
Gain a better understanding of Medidata’s latest technology. Strategize with our subject matter experts and leave with actionable insights and best practices. Grab a drink with colleagues and take in the views from the 65th floor of Rockefeller Center’s iconic Rainbow Room.
The Study Builder Revolution: Advancing Patient-Centric Clinical Trials
DCTs! Sensors! ePROs! All represent crucial elements to cultivating more patient-centric experiences in clinical trials. To get there, we rely on the extensive knowledge and expertise of investigators, clinical operations and regulatory professionals, patients, advocates, and everyone else in-between. There’s one key persona in the mix, however, that we should cast into the spotlight—the unsung hero laying the foundation of the multifaceted Medidata Patient Cloud user experience: the study builder!
Transforming Medical Coding and Data Review by Harnessing AI/ML and Automation
Life sciences companies are seeking ways to harness new technologies like AI/ML. There’s a mix of emotions ranging from excitement to hesitation about using these technologies. Let’s explore some of the challenges and benefits—while keeping in mind the goal of helping customers to be more efficient so they can focus on what matters most.
Knowledge Hub: What’s New
Medidata's Knowledge Hub now has a fresh, more accessible design and several new functionalities, including exporting table data. Check out the details in the latest Knowledge Hub blog post on our new design and features!
Clinical Minds Blog
Have you checked out the Clinical Minds Blog lately? Bookmark it and check back frequently for our latest insights and best practices.
Subscribe to MediVoice, our LinkedIn newsletter for the latest patient insights and clinical innovations.
Meet Our Subject Matter Experts

Tim Akers
Senior Director, Clinical Data Studio
Tim is passionate about solving the complexity and frustration that study teams have in their day to day workflows. He currently leads a global team of Subject Matter Experts focused on solving complex Central Data aggregation, standardization, and analytics needs. This group is focused on the agnostic approach to data aggregation, leveraging his past experience helping clients optimize their clinical portfolio. During his time at Medidata, Tim has also played a leadership role in the development of Medidata's vision for CTMS, eTMF, and RBQM solutions by helping clients design their personalized concept of platform products using innovative approaches to study team workflows.
His background surrounds various roles within the clinical research lifecycle. He has extensive experience as a CRA on multiple late stage trials primarily focused in Oncology. Tim also worked as a Project manager in Early Clinical Development. During his time at a large CRO, he helped develop strategic Vendor Management initiatives focusing on controlling and improving how business is conducted with 3rd party vendors in an outsourced clinical trial.
The Latest from Dassault Systemes
Live Webinar: Accelerate Batch Release in QC Labs
Tuesday, October 22, 10 AM ET
This webinar is designed to equip you with the knowledge and tools needed to revolutionize your QC lab operations. By attending, you will gain valuable insights into cutting-edge technologies and methodologies that can transform your lab's efficiency, compliance, and overall productivity.
Future Labs Live
Oct 30-31, Philadelphia, PA
Future Labs Live 2024 captures all elements of the laboratory, from fundamental scientific breakthroughs to the latest technological advancements, over two full days of collaboration, innovation, and networking. BIOVIA subject matter experts will be speaking, and be sure to stop by Booth #602.