From SHYFT Analytics to Medidata AI Commercial Data Solutions

A Little SHYFT Analytics History
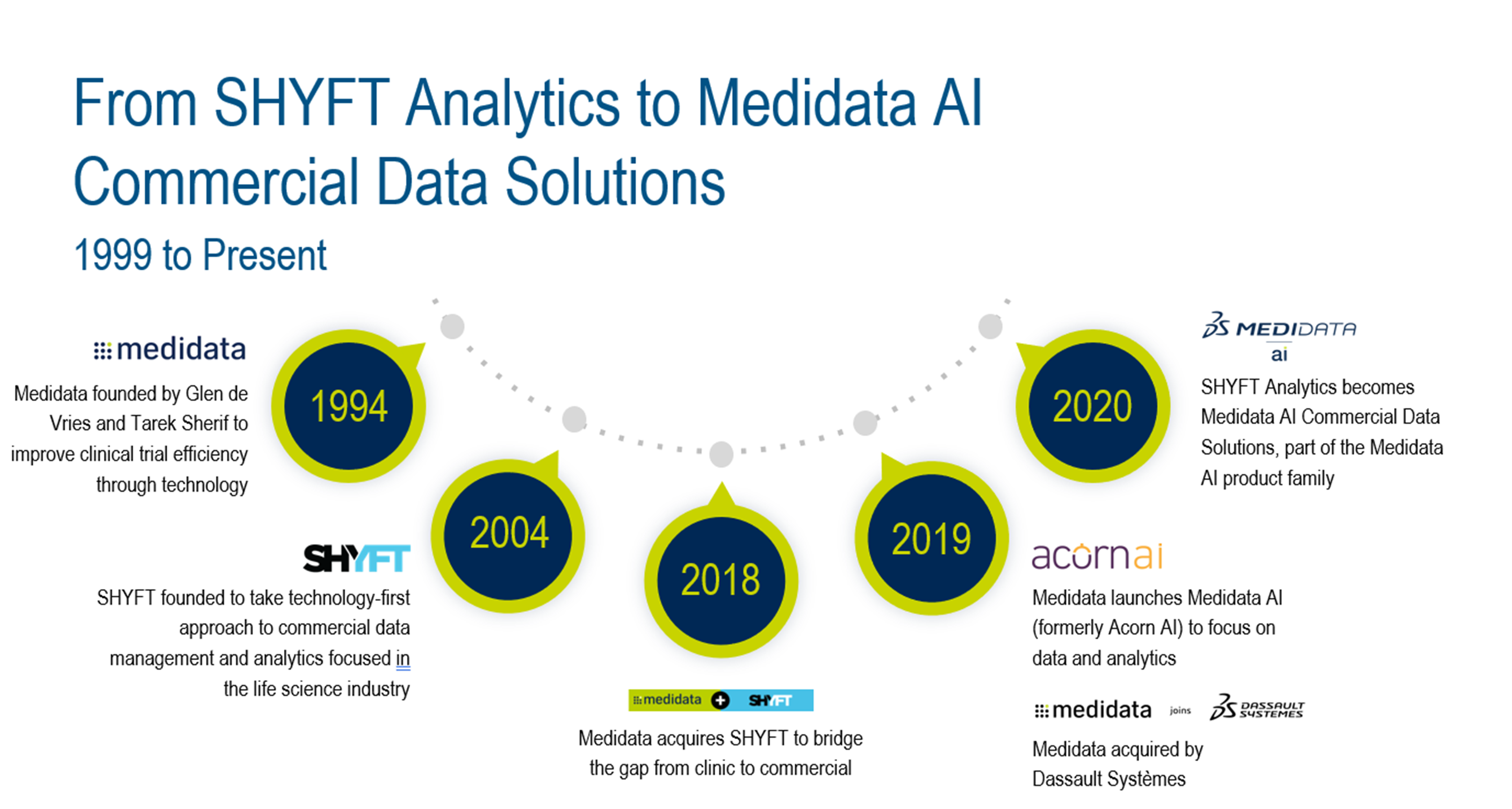
SHYFT Analytics was founded in 2004 by a team of technology and life sciences experts, including Frank Lane—currently Medidata AI’s vice president of Customer Engagement for Commercial Data Solutions. Over the next 15 years, SHYFT provided commercial data management and insights for over 75 brands across the industry and dozens of therapeutic areas. SHYFT also conducted countless real-world data (RWD) analyses that supported an array of real-world evidence (RWE) needs, including analyses for burden of disease and line of therapy. SHYFT was intent then, and continues today, to use a technology-first approach to solve the data management and insights challenges facing the life sciences industry.
In the mid-2000s, SHYFT served a growing category of drug manufacturers: companies launching their first drug product. From 2006 to 2018, the number of companies launching their first drug product tripled. According to McKinsey, this is in part due to the emergence of a new dynamic in the pharmaceutical ecosystem: vendors, such as SHFYT, that offered the manufacturers specific stand-alone commercialization capabilities.1 SHYFT was one of a small number of pioneering software-as-a-service commercial data management and analytics companies that gained a foothold during this period.
“When we started SHYFT, our mission was to enable emerging biopharmas and established pharmacos to harness the power of data and analytics as a competitive advantage, which was novel at the time,” says Frank Lane.
“Since then, we’ve continued to evolve the platform and our technology-first approach into something that really sets Medidata AI apart from alternatives. Our solution equips clients with near real-time insights that help guide tactical activities and enable value-add engagement with healthcare providers.”
In order for organizations to carve out a niche in the competitive drug manufacturing business, they needed to specialize themselves, in data management, for example. Emerging pharmacos didn’t need to be beholden to the deep pockets and distribution networks of established pharma anymore. Now, acquisitions and strategic partnerships still happen. In fact, acquisition may even be part of some business plans. But, this evolution in the industry allowed entrepreneurs at least a chance to see their hard work pay off.
Medidata Acquires SHYFT Analytics
In 2018, SHYFT was acquired by Medidata Solutions—the global leader in data capture for clinical trials. Medidata saw an opportunity to complement its clinical capabilities with SHYFT’s commercial capabilities, extending Medidata’s relevance into the commercial sector.
SHYFT was already using advanced analytics to empower emerging pharmacos with the intelligence needed to make critical, data-driven business decisions. For Medidata, SHYFT helped bridge the gap from clinical to commercial, making it possible to synchronize a client’s clinical trial data harmoniously and without interruption to their launch and commercialization data in one ecosystem.
SHYFT Analytics Becomes Medidata AI Commercial Data Solutions
In March 2020, while many people transitioned to working from home because of COVID-19, SHYFT Analytics began a transition of its own—it’s been just over a year since SHYFT became Commercial Data Solutions (CDS), part of the Medidata AI product family launched in 2019, finalizing the acquisition process. Though the name changed, the data experts, engineers, infrastructure, and technology that originated with SHYFT remain intact, continuing to leverage its universal commercial data model to provide commercial data management, master data management, specialty data aggregation, and insights for emerging biopharmas and pharmacos. Where Medidata AI is well known for its capabilities designing and monitoring clinical trials (among other functions), Commercial Data Solutions provides the commercial data management and insights for commercial operations once the drug is approved, completing the clinical to commercial journey.
Since the acquisition, Commercial Data Solutions has continued to see significant success and growth. As of June 2021, Medidata has over 1,800 customers, including global pharmaceutical companies, innovative biotech, diagnostic, and device firms, leading academic medical centers, and contract research organizations. Over twenty of those customers are supported by the Medidata AI commercial data management solution. In fact, the Commercial Data Solutions team supported the launch of more than a dozen new brands to the market since 2019. It’s clear that first-time drug launchers continue to see value in the stability and flexibility of Medidata AI’s innovative commercial data model and data management technology. Reliability is key when you often only have one shot at a successful commercial launch. The Medidata AI Commercial Data Solutions team has been implementing effective, industry-best commercial data management environments for more than 15 years.
Download our white paper to learn how Medidata AI's Commercial Data Model delivers on the promise of big data in pharma:
References
Explore Related Articles
Contact Us


