RBQM: The Connective Tissue for Data Quality

This blog was authored by Olgica Klindworth, VP, Data Quality and Risk Management Solutions at Medidata.
Let’s explore the significance of a harmonized risk-based quality management (RBQM) system by drawing parallels with the human body.
In today's clinical trial landscape, complexity is on the rise, with protocols becoming more intricate, leading to an influx of data and stakeholders. This complexity results in challenges such as data integration issues, the necessity for real-time data accessibility, and the demand for swift, data-driven decisions.
Similar to how the human body functions optimally only when all its parts work together, the RBQM framework serves as the connective tissue of various aspects of clinical trials.
So how can a harmonized RBQM system address these challenges in light of such complexity?
Data Quality Fueled by RBQM Principles
To achieve truly optimized data quality and ensure patient safety, the RBQM framework has to be a holistic approach—connecting people, processes, and real-time data.
Picture the risk management system as the brain, overseeing operations and decision-making. Quality by design (QBD) principles act as the immune system, safeguarding the trial's integrity. Like blood, data must flow seamlessly throughout the ecosystem, enabling stakeholders to make informed decisions swiftly. The platform acts as the heart and circulatory system, ensuring data flows smoothly to all parts of the ecosystem. Achieving data balance and stability within the RBQM framework is akin to maintaining homeostasis in the body—ensuring consistency and reliability in data throughout the trial.
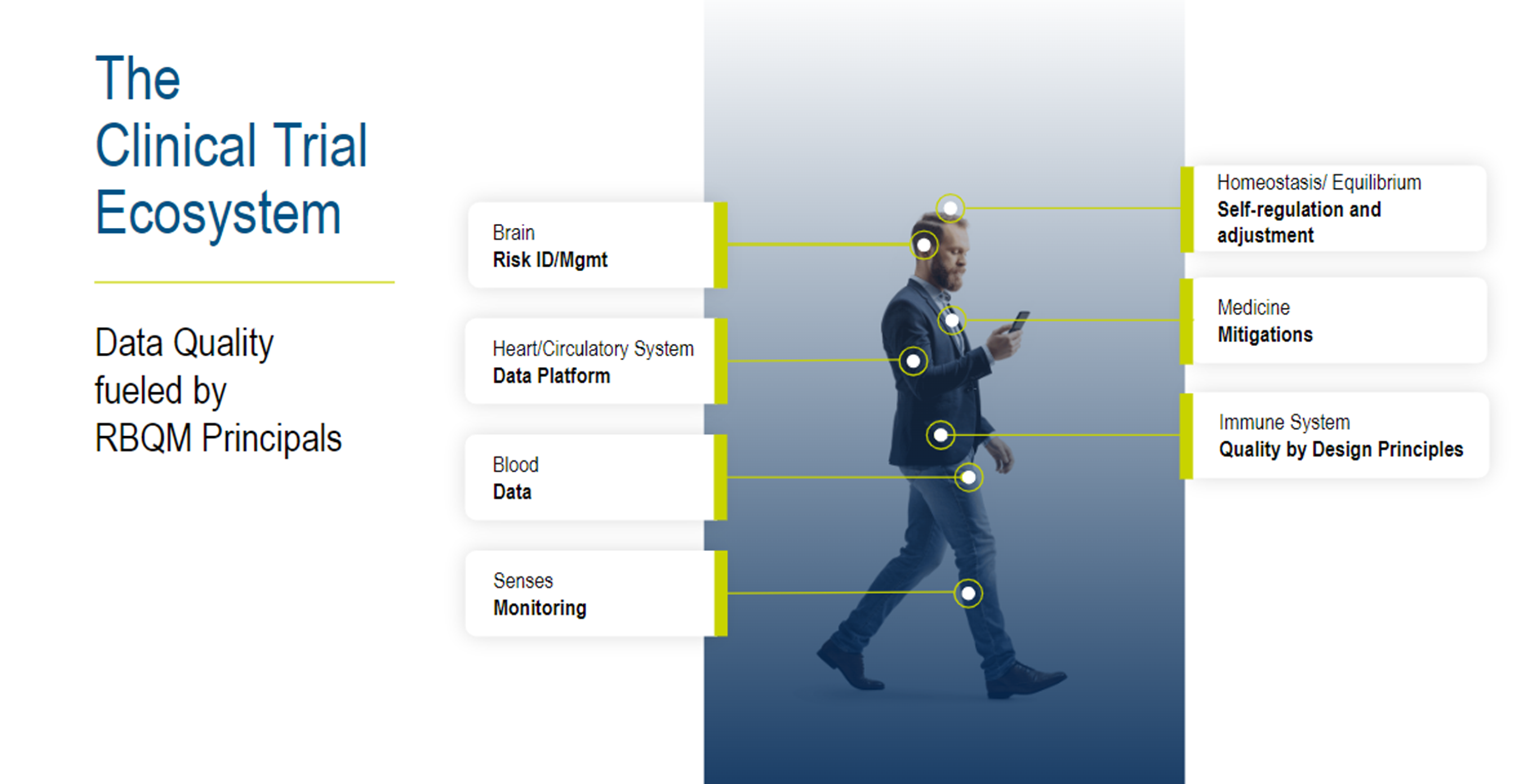
When a clinical trial operates under RBQM principles, it functions like a well-oiled machine, delivering quality, safety, and efficiency.
1. A Clinical Trial’s Immune System–QBD Principles
Imagine QBD principles as the immune system of a clinical trial, safeguarding its integrity and overall health, much like how our immune system protects our body from harm. The adaptive immune system learns over time and strengthens as it comes in contact with an antigen it already recognizes; this is our historical data, making our immunity stronger and better able to clear risk sooner from the system based on information it has from past encounters.
Just as a strong immune system ensures our well-being, QBD principles establish a robust foundation for trial success. This foundation is achieved by infusing quality considerations during protocol design and reducing the burden on patients and sites while enabling adaptability and reducing potential risks by leveraging historical knowledge.
By incorporating input from various stakeholders, QBD principles fortify the trial against potential pitfalls and risks, eliminating complexities and redundancies to ensure a solid risk strategy. And the various risk stakeholders should include sites and patients, to make sure all perspectives are considered—especially around two key concepts that are often on a critical path: patient enrollment and retention.
2. The Brain—Risk Identification and Management
Human brains have evolved from our primitive ancestors' brains–we don't rely just on the amygdala, the part of the brain responsible for processing base emotions like anger, avoidance, defensiveness, and fear; we also have a neocortex that lets us analyze risk.
Think of risk identification and management as the brain of an RBQM system, orchestrating visibility and understanding of protocol risks—similar to how our brain processes and analyzes information. Like the brain's memory, an RBQM system's central data repository enables continuous learning and adaptation, connecting with monitoring and mitigation strategies. It shares insights across the trial ecosystem, much as the brain communicates with various parts of the body, ensuring proactive measures to prevent the recurrence of issues.
Like the human brain, an optimized RBQM system collects, processes, and acts on information continuously—not just at predefined time points, e.g., monthly or quarterly.
3. It’s in the Blood–Data, Data, and More Data
Data serves as the lifeblood of an RBQM framework, akin to blood flowing through our veins, impacting the trial's functionality directly. Just as poor blood quality affects bodily functions, insufficient or incomplete data disrupts trial processes exponentially. Ensuring a constant flow of enriched, cleaned data throughout the ecosystem is vital for efficient risk monitoring, enabling stakeholders to make faster and better decisions.
Having continuous data requires a data-driven culture—another concept important in the context of RBQM—this includes data but also technology, organizational support, change management, and upskilling users.
4. A Trial’s Heart and Circulatory System—Data Acquisition and Aggregation
Data acquisition and aggregation act as the heart and circulatory system of a trial, ensuring a cohesive flow of information to all vital parts of the ecosystem. Similar to how the cardiovascular system pumps oxygenated blood, robust data platforms acquire and supply data seamlessly, supporting users with data quality responsibilities and insights through interconnected workflows and centralized access.
In the absence of a data platform where all data can be aggregated in real time, operational users are reliant on irregular data infusions and are unable to identify signals of risks early, similar to a body being on life support when attached to an extracorporeal membrane oxygenation machine.
5. A Trial’s Sensory System–RBQM Monitoring
Our body’s sensory system enables us to collect information about our environment, helping us to learn, identify risks, avoid issues, and improve. Supported by aggregated data and automated monitoring tools, RBQM stakeholders are empowered to identify risks faster.
Automation and AI already play a role in faster identification of potential data anomalies, analytics, and visualizations, e.g. QTL and KRI types of checks, help RBQM stakeholders identify localized vs systemic risks before they become issues.
6. A Trial’s Medicine–RBQM Mitigation
If risks were potential diseases, you don’t use the same prophylaxis for each one.
In the past, we used 100% source data verification (SDV) as a prophylaxis for every risk; e.g. there is a problem with site compliance, so let’s increase SDV to 100%. Today, we have data and we want to use that knowledge to apply smarter, targeted monitoring or precision medicine. Just as tailored precision medicine addresses specific diseases, RBQM mitigation today employs a layered, proactive approach to mitigate risks before they become issues. We flex our how we avoid disease; e.g., flu is most likely not a risk in the summer, so there’s no need for a flu shot or an extra load of vitamins.
But some diseases and risks are unknown or hard to uncover, and we have to add more advanced senses and checks via centralized monitoring. Depending on the risk, we increase the number of checks and frequency, observe closer, and—when needed—add supportive therapy or, when dealing with an inexperienced site, some more support, etc.
7. Data Balance & Stability–Trial’s Homeostasis
Data homeostasis within the RBQM framework ensures stability and consistency, much as a healthy body maintains internal balance. By adapting to changes and adjusting our data, site, and risk monitoring and mitigation, RBQM establishes a resilient environment, maintaining trial integrity throughout.
In Conclusion–An RBQM Ecosystem in Harmony
Much like the human body, a connected RBQM ecosystem is a sum of its parts. When in harmony, imagine an Olympian. When out of sync…well, that’s why we are all here, doing our part to help patients.
When a clinical trial is driven by RBQM principles, at its core there’s a data-driven culture and a connected system across a cross-functional framework. It delivers quality, safety, and operational efficiencies (costs, resources, timelines). Medidata Detect, our data quality & risk surveillance system, infuses RBQM principles into the Medidata Rave platform and services through a holistic approach, building in quality at every stage, applying proactive risk management, stakeholder alignment, a data-driven culture, and data homeostasis.
Contact Us

