Sustaining Healthier Life by Reducing Environmental Footprints | Medidata Sustainability Series

Medidata’s approach to sustainability is directly tied to its mission to power smarter treatments and healthier people. And because the health of our planet is closely intertwined with the physical health of its people, we consider environmental stewardship a core part of our business.
After all, if a life sciences company isn’t committed to sustainability, is it really committed to health?
From an environmental perspective, Medidata focuses its sustainability efforts in two areas:
- Reducing our company’s environmental emissions
- Helping our customers reduce their clinical trials’ emissions
Reducing Our Carbon Emissions
Medidata and our parent company Dassault Systèmes are working to reduce our carbon emissions. We submit annual assessments to the Carbon Disclosure Report (CDP), and set emission reduction targets that are approved by Science-Based Targets initiatives (SBTi). We are currently on track to achieve a SBTi TARGET of 52% in the next four years, which would be a 38% reduction from our 2018 levels.
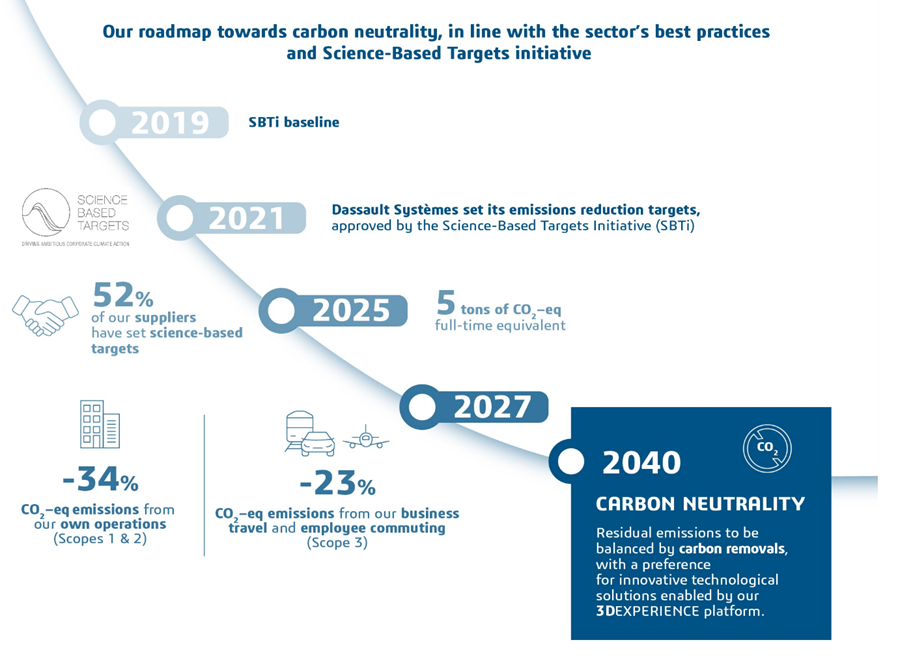
Medidata's roadmap towards carbon neutrality by 2040
More Sustainable Office Spaces
We have set the ambitious goal of having 90% of our long-term office leases in the U.S. be Green Building Council LEED Gold certified. We also encourage sustainable waste management and responsible energy consumption within all offices:
- Medidata is Energy Star rated.
- Datacenter in Frankfurt is supplied by 100% renewable energy.
- Our Data Center in Houston is LEED certified.
Reuse, Reduce, and Recycle Initiatives
As a technology-focused company, we are continuously evolving our tools to better serve customers and patients. Rather than discard equipment once they’ve outlived their usefulness to our technicians, we partner with NCS to recycle/reuse our IT waste. Doing so allows us to extend the useful life of some devices and re-deploys IT assets we cannot use but others can. This reduces the amount of waste in landfills and reduces the energy use and emissions associated with equipment destruction.
Helping our Customers and Life Sciences Industry
Beyond just our company, Medidata is committed to helping our customers—and the life sciences industry—be more efficient and sustainable through decentralized clinical trials.
Medidata’s solutions are unified on the Medidata Clinical Cloud®, with Medidata Rave EDC at the core, which allows customers to take parts of a trial—or the entire experience—outside a traditional physical clinic or trial site. Decentralization of their trials has shown signs of increasing sustainability, and we at Medidata continue to ingest data to learn more about the tangible impact this has:
- On average, trial patients spend two hours commuting to and from their physical test site each time they visit. Decentralized trials eliminate much of that travel, greatly reducing CO2 emissions.
- Many trials require patients to use multiple devices to log personal data, consuming fair amounts of energy. myMedidata, Medidata’s patient portal, gives patients access to their clinical trial needs through one web-based system. Patients can use any online device to learn, enroll, and participate in their clinical trial activities. This helps remove much of the need for a myriad of provisioned devices.
- myMedidata digitizes patient education materials and consent forms, saving paper.
- With Medidata Rave RTSM unified with Medidata Rave EDC, patient data is automatically randomized, automated, and controlled by an algorithm—rather than a traditional paper envelope—creating a more sustainable, secure, and reliable method of trial randomization.
- With our ePRO synced into EDC, patient data is captured digitally, removing the need for monitors to transcribe into traditional paper diaries. Also, for one of our largest pharmaceutical customers, our Global Diary and eDiaries will be used from study to study to eliminate waste for maximum trial effectiveness.
- Medidata Remote Source Review allows CRAs to virtually review source documents, reducing travel and increasing time efficiencies.
- With Medidata Rave RTSM, direct-to-patient shipping reduces carbon footprint and electronic supply accountability enables better waste recovery. RTSM also enables supply optimization & forecasting to reduce waste with evidence of responsible destruction.
- Medidata’s Synthetic Control Arm® reduces energy consumption by lowering the number of patients needed for a trial. This in turn reduces patient burden and carbon emissions caused from traveling to a site, while increasing the chance that more patients will receive the experimental therapy.
At Medidata, we’re 100% committed to improving global health, which includes the health of people and the planet we all share. Our continual–—and constantly evolving approach—for improving our technology solutions and practices will help us make an impact in both the short and long term.
Learn more about Medidata's commitment to sustainability in Part 1 of this blog series.
Explore Related Articles
Contact Us

