The Role of Source Data Verification (SDV) and Source Data Review (SDR) in Driving Clinical Trial Data Quality

Like many business processes, clinical trial operations require planning, compliance with regulations, and coordination between multiple stakeholders. Risk-based quality management (RBQM) of clinical trials is a targeted, strategic approach that supports project teams to focus on what matters most and thus place resources in the areas that bring the most significant value. The aim of RBQM is to focus monitoring and oversight activities on those trial processes most likely to affect participant safety and data quality, to enable clinical operations teams to mitigate risks or address errors quickly and effectively before they compromise trial outcomes. A significant component of RBQM is reduced source data review (SDR) and source data verification (SDV).
A considerable amount of time and cost from clinical trials are attributed to manual monitoring–an industry paper estimated 46% to 50% of time is attributed to 100% SDV and an average of 25-40% of clinical trial costs.1
TransCelerate BioPharma’s landmark position paper (2013) about risk-based monitoring (RBM) determined that only 2.4% of the queries in critical data were driven by SDV.2 Other sources concur and make similar observations, demonstrating that this approach is unsustainable–up to 3% of all case report forms (CRFs) are attributed to data changes due to 100% SDV, allocating over 50% of site monitoring budgets, and spending up to 50% of time carrying out 100% SDV on-site.3
What Is Source Data Verification (SDV)?
By definition, SDV is the process of ensuring that the data reported for analyses accurately reflect the source data at the clinical trial site, a comparison of source data against the case report form data (transcription errors). SDV predominantly detects random errors that likely have little impact on the results of clinical trials.
What Is Source Data Review (SDR)?
Source data review is the review of source documents in relation to the clinical conduct of the protocol. SDR focuses on areas that may not have an associated data field in the CRF or a system. Historically, SDV has been conducted for most of the CRF data; however, SDV of 100% of the data does not guarantee error-free results, and concentration on transcription accuracy does not guarantee data quality. SDR instead focuses on the quality of data collection and compliance against the protocol and standard of care. As a result, SDR tends to be more strategic, resulting in a heavier focus on the present and future proactive activities to maintain data quality.
Designing Quality into Study Protocol & Processes
In the European Medicines Agency’s (EMA) International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) “Guideline for Good Clinical Practice” (GCP), one of the key themes is alignment with quality-by-design (QbD) principles in clinical trial planning.
It was made clear that the overall quality of a trial is driven proactively by designing quality into the study protocol and processes, with appropriate and fit-for-purpose use of technology.
Therefore, the most accessible and most significant value gain is from a targeted and focused approach to reduced SDV and SDR.
The Shift from 100% SDV to Risk-based Monitoring (RBM)
With less than 3% of queries in critical data being driven by SDV, the conclusion is that SDV has a negligible effect on overall data quality. Additionally, the impact of SDV on a study is wide reaching–reducing SDV would positively impact data quality, data integrity, compliance, and costs, while decreasing operational efficiency, improving assessments for risks and critical quality factors, improving data management, and positively impacting trial outcomes.
Whilst attitudes to adopting RBQM are predominantly positive and implementation of RBQM technology solutions has increased since 2020, there are still barriers to industry-wide adoption (Figure 1).
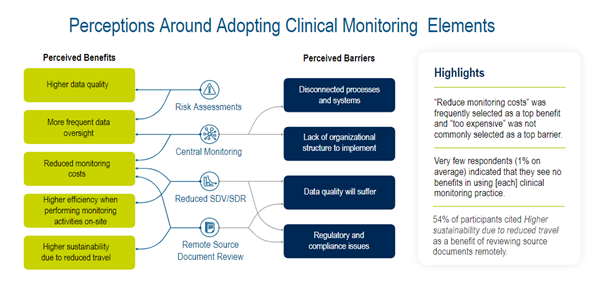
Figure 1. Perceptions around adopting clinical monitoring elements.
In conclusion, as clinical trial operations continue to evolve, monitoring and oversight activities should focus on trial processes most likely to affect participant safety and data quality to address errors quickly and effectively, before they compromise trial outcomes.
Evolving Regulatory Guidelines for RBM Adoption
Guidance to adopt RBM practices was given by regulatory authorities in 2011, and notable updates and new guidance have been published since 2013.4
Two excerpts are shown here:
“Evolutions in technology and risk management processes offer new opportunities to increase efficiency and focus on relevant activities,” and “Advances in use of electronic data recording and reporting facilitate implementation of other approaches. For example, centralized monitoring can now offer a greater advantage, to a broader range of trials than is suggested in the original text. Therefore, this guideline has been amended to encourage implementation of improved and more efficient approaches to clinical trial design, conduct, oversight, recording and reporting while continuing to ensure human subject protection and reliability of trial results.” (ICH E6 (R2) 2016)
“Quality by design in clinical research sets out to ensure that the quality of a study is driven proactively by designing quality into the study protocol and processes.” (ICH E8 (R1) 2021)
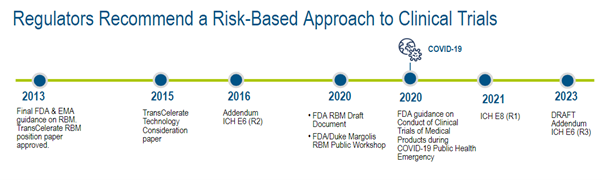
Figure 2. Regulator timeline.
Risk-based quality management (RBQM) is a methodology and strategy that has a more expansive approach than RBM, involving a continuous cycle that consists of planning and initiation, identification and assessment, management and control, and implementation and adapting. A significant component of RBQM is reduced SDV and SDR.
The methodology and deployment of advanced RBQM technologies provide a targeted, strategic approach that supports clinical trial project teams, reducing burdens while enabling them to focus on placing resources in the areas which bring the most significant value.
RBQM has played an increasing role as the industry continues to focus more on DCT. In a Medidata commissioned industry survey, the elements that sponsors stated are most needed for operationalizing monitoring in DCTs are risk assessments (57%), remote source document review (40%), central monitoring (26%), and Reduced SDV/SDR (26%). (Figure 3)
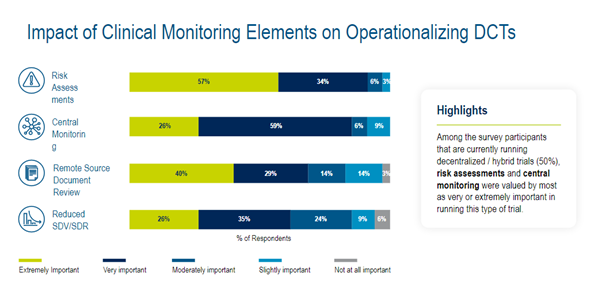
Figure 3. Impact of Clinical Monitoring Elements on Operationalizing DCTs.
Effective Alternatives or Additions to 100% SDV
As RBQM methodology and technology has evolved, clinical trial organizations have shifted from remote source data verification activities to more strategic quality management methods, such as remote source data review. SDV should be considered one of many potential quality control mechanisms used to determine whether an acceptable level of accuracy has been achieved in the transcription of critical data. However, a heavy reliance on SDV should not be taken as a mechanism to ensure study quality oversight. To determine the proper volume and targets for Reduced SDV & SDR, a critical first step is a protocol-based risk assessment to inform an intelligent monitoring strategy, followed by the introduction of strategic quality management methods: Reduced or Targeted SDV, remote SDR, and centralized statistical and data monitoring.
Targeted Source Data Verification (TSDV)
Targeted Source Data Verification (TSDV) activities can be easily established using critical-to-quality factors to identify data and processes most likely to impact trial results. These activities are best conducted within a unified platform to ensure sound audit readiness and efficacy.
The variability in global regulations, standards of care, and technological infrastructure across different countries adds additional layers of complexity that cannot be addressed by a one-size-fits-all approach, requiring instead strategies, technologies, and services that are highly flexible and able to mirror the nature of complex studies.
The Future of SDV & SDR
Assessing RBQM technology in line with current and future needs can be complex.
The Medidata survey highlighted expected trends in monitoring component utilization, showing a shift to central monitoring, remote source document review, and risk assessments in particular. (Figure 4)
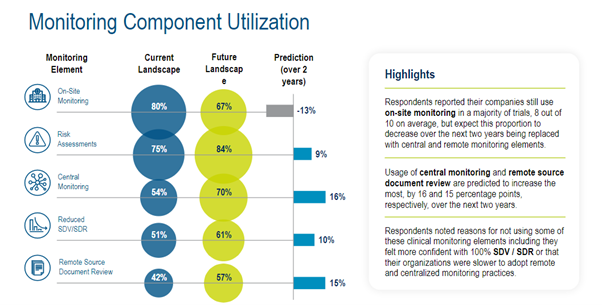
Figure 4. Monitoring component utilization.
The best option is to use fit-for-purpose solutions that sit on a unified, advanced technology platform designed to enable seamless cross-communication internally and with third-party systems and data sources.
One possible solution is the piecing together of disparate technical solutions to fulfill the different elements of an overall RBQM ecosystem, but industry feedback shows that integration and interoperability are two of the most prominent issues faced when that approach is taken. This is because the different systems used often have difficulty interfacing with at least one other system in a seamless way. This leads to delays, inconsistent user experiences, burdens on sites and patients, costs, and inefficiencies.
The future is clear–an appropriate RBQM technology platform empowers clinical trial teams to overcome the many challenges they face today and tomorrow.
To learn how we have helped sponsors and what we can do for you, contact us here.
References
-
-
- Hamidi M, Eisenstein EL, Garza MY, et al. Source Data Verification (SDV) Quality in Clinical Research: A Scoping Review. Journal of Clinical and Translational Science. Published online 2024:1-33. doi:10.1017/cts.2024.551
- TransCelerate BioPharma, Position Paper: Risk-Based Monitoring Methodology, 2013
- E. Eisenstein, P. Lemons II, B. Tardiff, K. Schulman, M. Jolly, R. Califf, “Reducing the costs of phase III cardiovascular clinical trials” American Heart Journal 149, 3 (2005): 482-8.
- EMA, GCP ICH E6 (R1), 2002 ;EMA, GCP ICH E6 (R2), 2016; EMA, GCP ICH E6 (R3), 2023; EMA, ICH E8 (R1), 2021
-
Explore Related Articles
Contact Us

