Preparing for a New Data Future: Examining Industry Outlook on eClinical Platforms
This blog is part one of a five-part series on the findings of this survey of clinical research technology decision makers. Download Preparing for a New Data Future: A Survey of Clinical Research Technology Decision Makers for comprehensive survey results to guide your unified platform approach to clinical trials.
A rapid shift towards virtualization due to the COVID-19 pandemic, larger volumes of data collection from an increasing number and diverse set of data sources, and the need for sponsors and CROs to gain more complete pictures of trials and to act on data quickly are forcing the industry towards eClinical software solutions that are on unified platforms. Our new survey of key decision makers in clinical research technology examined industry outlook on eClinical platforms. Key findings include:
Trial optimization will be analytics driven
Artificial intelligence (AI) and machine learning (ML) increasingly inform critical clinical trial decisions such as study design and site selection. These analytical tools address the need to synthesize insights from diffuse data across clinical trials as data gets ingested from an expanding range of sources, including wearables. AI and ML systems—which reduce trial time, optimize study design, and allow for new virtualization methods—depend on unified data.
Clinical trials will incorporate more virtual elements
The industry measuredly trended toward more virtual clinical trials in the past few years. The cautious pace of adoption, of course, has been dramatically accelerated by the COVID-19 pandemic. While trials components require patients to visit sites regardless of the situation, the pandemic has forced companies to accomplish as much as possible through virtual methods.
Virtual trials—also referred to as decentralized, hybrid, or digital trials—where one or more patient-facing trial activity that typically takes place on-site is conducted off-site through virtual methods, now make up an increasing percentage of all studies. Approximately 35% of healthcare experts are using decentralized trials and 67% planned to use them in the future, according to a June 2020 report from GlobalData. While trials with virtual elements—where some on-site components are still required—are making up a greater percentage of all trials, studies that are conducted entirely off-site using fully virtual methods remain rare.
Virtual trials benefit both sponsors and patients by increasing the size of patient pools, decreasing patient burden, and enhancing patient retention. As patients participate in trials more remotely, companies need software capabilities to meet these changing needs.
“[Growth in virtualization capabilities] was being done methodically, [but] COVID, as you can imagine, has given us a good, swift kick in the rear to ensure that we are moving at a heightened pace”
—Former director, health outcomes and market access, large life sciences
IT overhead costs will decrease through outsourcing
The industry sees more of their legacy IT overhead and maintenance costs being replaced by vendors’ cloud-hosted software-as-a-service (SaaS), and there will be more outsourcing of either individual trials or specific clinical functions.
Clinical data will be better unified
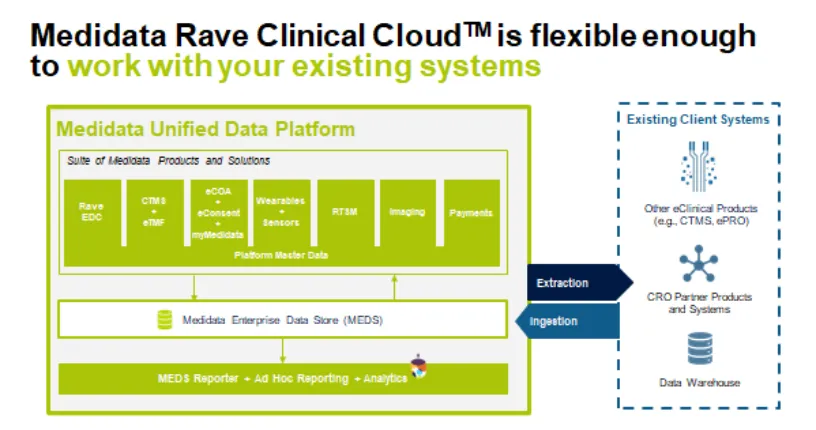
Due in large part to poor cross-application data sharing, data analysis and reporting remains a challenge for companies ingesting data from different sources with different system architectures and data structures. Often data aren’t unified because sponsors tend to cherry-pick best-in-class point solutions (eCOA, ePRO, EDC, etc.) for different parts of a clinical trial. This approach creates a system with many disparate applications that do not work together or cross-share data.
Consequently, stakeholders can’t view data across their trial from start to finish and can’t unify disparate data streams to generate novel insights. In fact, 75% of respondents assessed the “availability of data across all clinical products” as a 9 or 10 on a 10-point scale intended to measure the most important drivers of adoption for unified platforms. Survey results suggest that the advantage of the “best-in-class” for eClinical software applications is narrowing and the opportunity for platform solutions that can unify cross-application data is growing.
“Clinical data [availability] is really the gold dust… if you [are able to] access that data, however big or small you are, the sooner you can make decisions on next phase, next regulatory approval process, reporting out to publications… all of those things.”
—Former head of biometrics, mid-sized CRO
The need for “best-in-class” will decrease
Respondents indicated that companies choose the best point solution for their needs, with 70% of respondents saying they consider the “strength of a specific eClinical solution” as a 9 or 10.
However, this is changing based on responses that indicate sponsors are thinking about factors that go beyond a specific application—such as data unification, data surfacing, and interoperability—which suggests that companies are balancing application strengths with unified platform capabilities.
According to multiple respondents, eClinical solution providers with reputations for single or specialized applications are seeing their market advantages shrink. The market landscape is changing because “best-in-class” the differences among offerings from competing providers is narrowing to a negligible degree.
The value of “best-in-class” solutions brings diminished value compared to the ability to connect applications, which a unified platform achieves by design.
Stay tuned for the next edition of this five-part series. Download Preparing for a New Data Future: A Survey of Clinical Research Technology Decision Makers for comprehensive survey results to guide your unified platform approach to clinical trials.
Contact Us

