Practical Applications of AI in Imaging Clinical Trials

Automation is improving virtually every stage of the clinical trial imaging workflow. Sponsors can benefit from improved compliance with privacy regulations, stronger data quality controls, more accurate and efficient imaging reads, and advanced data analysis for improved decision making. This article outlines three very practical applications for AI in imaging for clinical trials around PHI detection and removal, image quality control and reviewer assessment.
PHI Detection and Removal
Data privacy regulations in the US Health Insurance Portability and Accountability Act (HIPAA) and in the EU (GDPR), require that personal health information (PHI) be redacted from clinical trial data. PHI must be stripped from medical image files before they can be transferred from clinical trial sites to a central lab, contract research organization (CRO), or sponsor. While investigator sites are required to do this, it can be a time consuming and error prone process.
Such incoming data needs to be double-checked and scrubbed of any PHI that was not caught by the site. Traditionally, this second review has been done manually by an imaging core lab or other designee. However, Medidata’s ByePHI AI algorithm will automatically detect and redact PHI from Digital Imaging and Communications in Medicine (DICOM tags) and pixel data before it’s transmitted to other users. This algorithm, available in Q1 2021, will help ensure compliance with all data privacy regulations and remove any opportunity for human error in the review step.
Image Quality Control
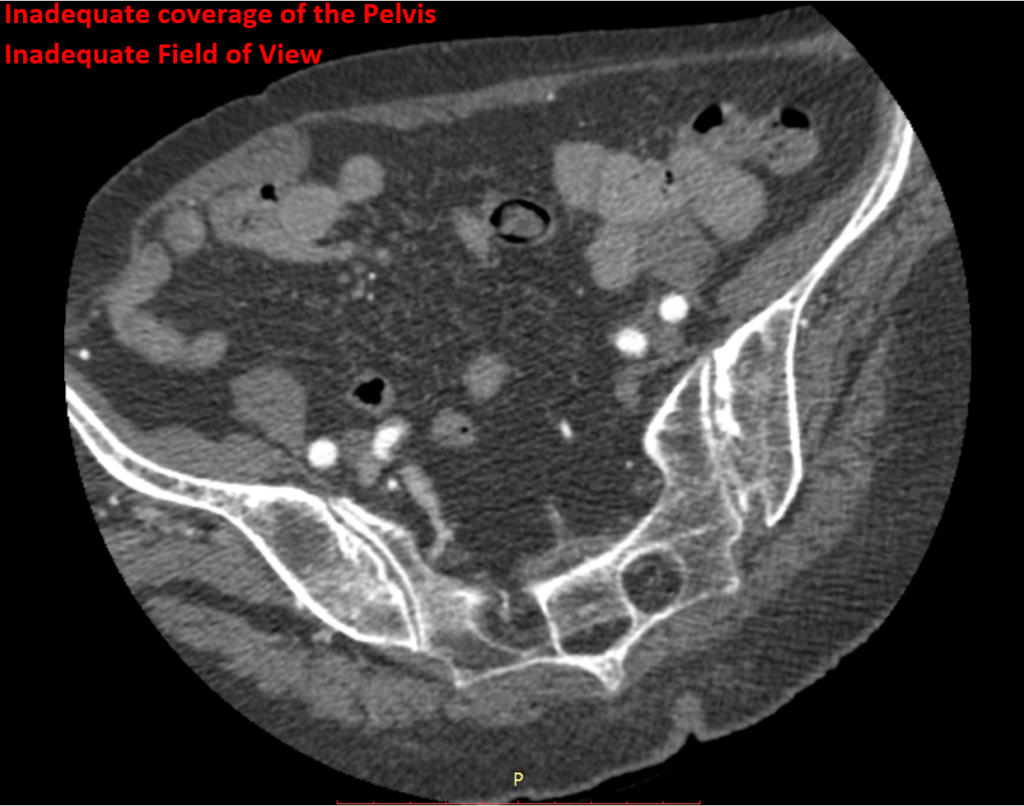
The standard quality control process for medical images generated in a clinical trial is for incoming images from trial sites to be reviewed against a variety of quality control parameters. For example, in a standard oncology trial, the following parameters are typically confirmed: anatomical coverage, contrast enhancement, scan parameters, field of view, and patient consistency over time. These parameters are critical to ensure a subsequent accurate assessment by a radiologist, to collect clinical trial endpoints of good quality, and to avoid unevaluable data.
This is a manual, subjective, and time consuming step in clinical trials. To help remove subjectivity and reduce the amount of time a user needs to spend performing manual QC, Medidata is creating an AI algorithm that automatically detects critical quality parameters such as proper anatomical coverage, consistency among patient imaging across visits, and presence of contrast enhancement, among other important checks.
Reviewer Assessment
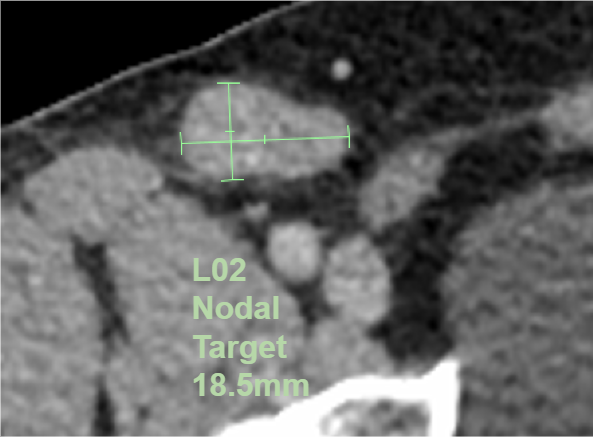
Imaging assessments remain critical to support endpoints for oncology trials. However reviewer assessments can be expensive, time consuming, and prone to variability amongst readers. To address these issues, Medidata and Dassault Systèmes are partnering to develop an algorithm to aid radiologists in their oncology assessments by performing automated measurements of lesions according to the relevant assessment criteria (RECIST, Lugano, RANO, etc.).
The algorithm will be integrated with Rave Imaging and will identify, premeasure, and categorize lesions for presentation to the radiologist as shown. Radiologists would then review the algorithm’s work and either approve it or modify it as they see fit. This would save time and money, as radiologists can be one of the trial’s most expensive resources. Just as important, it will also reduce the variability between readers by presenting them with consistent lesion identification. This algorithm will be available in 2021.
The advances discussed here could transform not only how imaging data for clinical trials is collected, processed and analyzed, but also how the imaging data is cleaned and correlated with clinical outcomes. Read more about AI and imaging in our white paper Clinical Trial Imaging - 2020 and Beyond.
Contact Us