Identifying Risks in a Tidal Wave of Clinical Trial Data

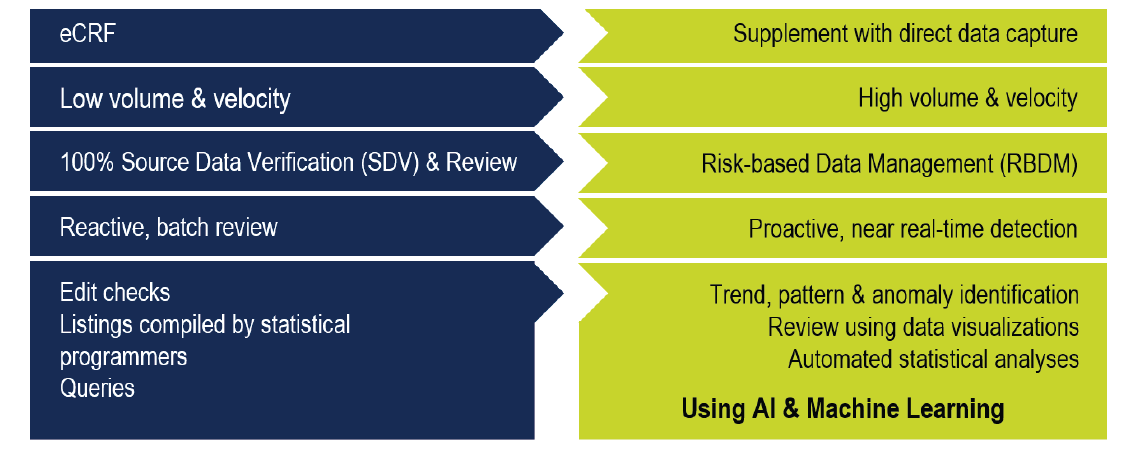
The rising complexity of clinical trials has created great challenges to their traditional clinical data management process. These challenges present new obstacles to clinical data management, but they also create the opportunity to apply risk-based quality management (RBQM) principles to clinical data management. Risk-based clinical data management (RBDM) has been rapidly expanding over recent years. This trend is expected to continue, especially as trials continue to incorporate more high-volume, high-velocity clinical data from a wider array of sources.
Traditional clinical data management processes lack the necessary modern, adaptive, and intelligent analytics. So it comes as no surprise that sites, sponsors, and clinical research organizations (CROs) have been progressively implementing RBDM approaches. Traditional clinical data management has historically used burdensome and time-consuming processes that strived to ensure that 100% of trial data was collected, missing data retrieved, and inconsistent data cleaned. However, given that only a small percentage of data are changed after entry, the vast majority of edit checks are not triggered and query-based data review has been shown to be ineffective, modern risk-based approaches to clinical data management focus on high-impact data points to achieve specific data integrity goals that are fit for purpose.
“It’s okay not to be perfect. Every data point is not equal, and we should prioritize data points that have the highest impact.”
– Wayne Walker, SVP, Rave Platform Technology, Medidata
By using an RBDM approach, you deploy scalable and intelligent processes to provide proactive and continuous virtual oversight of data quality in near real-time. This strategy allows you to identify unusual trends, patterns, and outliers quickly, before they become a systemic problem that can stifle trial progress and delay database lock. An RBDM approach, supported by near-real-time, intelligent analytics reduces edit checks by 20%–40% and allows for automating 50%–55% of data reviews. Traditional data review, cleaning, and reconciliation have meant that the final database lock has taken many weeks. Modernized clinical data management processes can accomplish it in hours or days since ongoing intelligent and incremental task completion leaves fewer tasks to complete after the last patient, last visit.
Risk-Based Approach for Modern Clinical Trial Data Management. Traditional Methods Do Not Scale.
Case Study: Identifying Data Issues in Very Large Data Sets
In a recent “mega-trial” designed to investigate the safety and efficacy of a novel anti-SARS-CoV-2 vaccine, an intelligent and automated analytical platform that uses RBDM was deployed to detect underreporting of adverse events (AEs) in near real-time. Despite rapid enrollment and high volume and velocity of data collection, the RBDM approach successfully identified missing AE data very quickly. The technology triggered a deeper analysis that showed that missing AE data was due to incomplete eCOA diary entries. Since the missing data were identified in almost real-time, they were quickly rectified. The sponsor was able to avoid compromising the statistical power of the study. Due to the sheer size of the trial, combined with the volume and velocity of data collection, traditional clinical data management processes would not have been able to support the real-time review and analysis that was required by this important trial.
Summary
As clinical trials continue to rise in complexity, an RBDM strategy will be key to adapting and succeeding in the modern clinical trial landscape. By proactively monitoring clinical trial data quality, RBDM allows data quality issues to be addressed quickly, which ultimately reduces the risk of incorporating poor data quality, while maintaining study integrity, and patient safety.
Download our white paper to learn how to succeed in an increasingly complex clinical trial world:
Contact Us

