Receive Investigational Medicinal Product at Your Doorstep – How Global Regulation Safeguards the DtP Process

In the modern world, we are no strangers to home deliveries. From our groceries and garments to gadgets and credit cards, we are used to the luxury of typing in our home address and waiting for the doorbell to ring—here is our package!
With the technological evolution penetrating every aspect of our days—and significantly simplifying our lives—clinical trial patients can also benefit from a more decentralized approach to trials, with procedures becoming more seamlessly integrated into their normal daily lives. While previously a patient had to travel to a site to collect the IMP (investigational medicinal product) or supplies from the investigator in person, now they can receive the IMP from the comfort of their home with direct-to-patient (DtP) delivery of IMP.
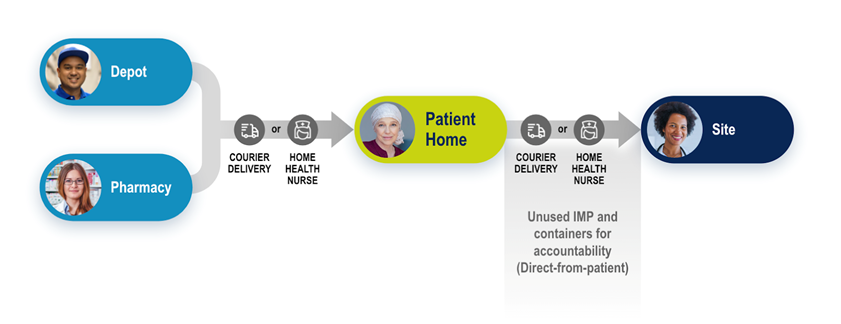
DtP can take many delivery modes, but the driving principle is that the IMP or clinical trial supplies are delivered and personally handed to the patient or their caregiver at home. There are options for how the IMP makes its way to the patient, including direct delivery by site staff using a third party courier, local pharmacies, or home health visits from nurses.
The COVID-19 pandemic has demonstrated how switching to DtP can be a necessary, enabling step to preserve clinical research and keep patients on treatment during unprecedented circumstances. Global regulators have, on the whole, demonstrated a great level of flexibility with guidance documents for the conduct of clinical trials during the pandemic; these documents outline alternative procedures and considerations, including—in many cases—DtP. These guidance documents were published by the EMA, numerous EU member states, FDA, Canada, Australia, Singapore, and many other countries globally, and contain considerations for DtP processes.
However, DtP has existed before the pandemic, and COVID-19 has become the catalyst step for adoption in some areas. For example, the MHRA have published DtP best practices in their MHRA GCP Guide back in 2012, demonstrating how the DtP process has been welcomed by the UK regulator long before COVID-19.
In some world regions, COVID-enabled regulatory flexibilities were key to facilitating a transition to a DtP model. Coincidentally, last year we have seen the emergence of decentralized clinical trial (DCT) guidance documents, which mention considerations for DtP, and remove the limiting condition for DtP processes to be permitted as exceptional only for the duration of the pandemic. Denmark pioneered the DCT guidance field with a first-of-a-kind document, and now the Danish National Centre for Ethics has published a DCT guidance. SwissMedic and SwissEthics have also released a DCT guidance, and Sweden has a set of considerations on their website. The documents provide a comprehensive overview of the key regulatory points to consider for a DtP study.
Main regulatory consideration themes for DtP are echoed amongst regulators. For introduction and overview purposes of this blog on the topic of regulatory considerations of DtP, common themes can be identified as key considerations for a DtP study set-up.
These areas are:
- Assessing the appropriateness of DtP of the IMP, including for home storage and administration, and in transit stability and integrity, including temperature checks.
- Setting up of concrete logistical workflows, which ensure patient confidentiality, and that the IMP is delivered personally to the patient or carer, and is not left at the door or with neighbors.
- Training patients on storage and administration according to protocol, as well as establishing a frequent communication line to monitor compliance with protocol, and wellbeing and safety of the patient.
- Monitoring accountability and returning unused IMP and containers back to the investigator.
Medidata and the Global Compliance and Strategy team continue monitoring the evolving global regulatory landscape of DtP. A recent Medidata webinar explored the important factor to take into account when planning a DtP study.
Watch our webinar to learn more:
Explore Related Articles
Contact Us


