Get The Return You Need On Your mHealth Investment
You just decided to invest a significant portion of your innovation budget to power your clinical trial with new mobile health (mHealth) technology. Now what?
Wearable sensors, apps and other mHealth tools offer the possibility to generate new types of data and massive amounts of it, but that only matters if you’re getting high quality data that is comprehensive, continuous and attributable to the source. No one needs a terabyte of bad data.
The influx of mHealth tools is shifting the paradigm of clinical trials towards more objective rather than subjective data; for example, using an activity tracker, we can measure how much a person actually walks each day rather than asking them how much they think they walk.
When pharmaceutical companies and medical device companies spend millions of dollars on these tools, they are making an investment in their data. So how do you ensure a high return on your investment?
The evolution of wearable sensor technology has only just begun. Pharmaceutical and device companies, investigators and regulators are all figuring out how to best implement mHealth technology in clinical development.
Those that lay the groundwork now by investing in infrastructure and training to support mHealth-enabled trials will be far ahead of the competition in the next five to 10 years, when mHealth is pervasive in clinical research.
Here are a few considerations to keep in mind as you prepare to roll out an mHealth-powered clinical trial:
Which trial makes sense? Equipping clinical trials with wearable sensors and apps only makes sense for the right trials. mHealth technology has the potential to track a wide array of physiological and behavioral data in a real world setting, but each component should be evaluated for fit and purpose.
Sensors can not only measure traditional in-clinic vitals such as blood pressure, heart rate and skin temperature, they can also monitor quality-of-life data such as patient movement or sleep and can be combined with apps that provide a continuous understanding of patient response to therapy. Make sure you can answer how this technology will be able to support your trial endpoints.
As the appetite for mHealth technology in trials continues to grow, we have seen interest in sensors and apps for use in anything from measuring sleep for cardiology studies to determining cognitive dysfunction in neurology trials.
Do you have the right partners to support your trial? Not all sites and clinical research organizations (CROs) are created equally. It’s vital to ensure your sites and CROs are able to support mHealth technology throughout your trial. Make sure they are trained with the skills necessary to instrument patients with devices and apps, and make sure they know how to handle data as it comes in from the patient. Don’t let your new data languish behind an unprepared site or CRO.
Your sites are your eyes and ears on the ground, so having sites that buy in to the promise and excitement of mHealth will go a long way towards ensuring a successful study. It’s important to equip sites with tools to monitor if and how patients are using their devices. The patient may not know they are using their device or app incorrectly, and proper monitoring can catch inadvertent user errors. In some instances, the site may need to provide basic support, or at least facilitate the patient getting the help they need.
How will you get mHealth devices to your patients? Sensors and apps may be prevalent in the consumer market, but implementing these tools in a clinical trial setting requires meticulous planning, budgeting and execution. The bring-your-own-device (BYOD) model is in its early stages, and if not all of your patients have a smartphone to use your app, you may have to provision one for them, and that’s expensive.
Don’t underestimate the complexity of global provisioning. Logistics for device purchasing, shipping, managing global data and issuing replacements are complicated. Luckily, vendors exist to take these logistic operations off your plate, which allows you to focus on your core strengths – finding cures for waiting patients.
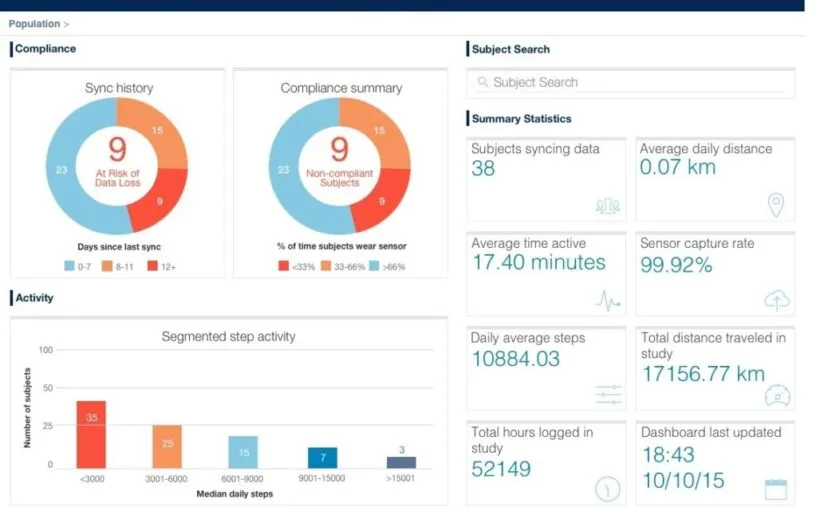
How will you ensure you get the data you need? Sensors and apps only work if patients use them. Options such as push notification reminders can keep patients engaged throughout the trial, and operational reports can keep you informed of their behavior (see below for a screenshot from Medidata’s dashboard). Compliance is key.
It is important to think about how the patient can go about their day unobtrusively when using a sensor or app. For example, devices with a long battery life can help ensure patient compliance, because patients won’t have to remember to charge them. For patient populations less familiar with smartphones, a solution that syncs data via a hub might make more sense.
Data from sensors can be sent back to a platform that will provide sponsors feedback on how frequently and consistently patients use these tools.
It’s not just about new clinical insights… It’s vital to be able to draw operational observations along with the clinical insight.
Develop a hypothesis on how the device will work in the trial. What are your expectations for the tool’s impact on patient compliance/engagement? Make sure you’re able to collect data on the tool’s operational performance. No matter how a hypothesis pans out, you’ve gathered highly useful data to inform future trials.
By understanding the issues around the operational aspects of the trials, you can implement best practices for your next mHealth-enabled trial.
These are early days for mHealth trials. Challenges still need to be addressed, and organizations are testing ways to fit mHealth into their trials, but it’s clear that life science companies see the benefits of mHealth. There’s no doubt that this technology is here, and it’s here to stay.
Learn more at Medidata:
Contact Us
