Is the Future of Clinical Data Management Finally Here? Indeed, It Is! | The Future of Clinical Data Management Series

This blog was authored by Nicole Pollard, Principal Engagement Consultant, Strategic Consulting, Medidata.
The future of data management has arrived. It is currently being modernized by the unification of data and workflows on cloud-based platforms to improve data management systems’ scalability, flexibility, intelligence, and interoperability with a variety of third-party systems. Explore this post to learn where your organization currently sits within the clinical data management landscape and about the changes that must be implemented to minimize your data management risk and to optimize your cleaning strategies to support novel trial design paradigms, such as decentralized clinical trials which are also explained in this post. Deploying modern clinical data management approaches can make all the difference when it comes to ensuring your drug development programs are future proof.
The Changing Role of the Clinical Data Manager
Over the last decade, stakeholders in clinical development have touted the changing role of the “Clinical Data Manager.” The underlying message is that clinical data management needs to change and must do so quickly to keep up with the increased complexity of clinical trials. Trials have evolved to include an ever-expanding array of data sources (e.g., wearable devices or sensors), increased data volume and precision, risk-based quality management techniques, decentralized clinical trials, and adaptive designs. Further, clinical trials are placing greater emphasis on the patient experience and value differentiation, which are additional factors contributing to increased trial complexity. Together, these factors have ultimately disrupted the old-school, heads-down data cleaning methods that clinical data managers were enabling and executing. The future of data management is here, and modernized processes and technologies are available to support clinical data managers in their roles and responsibilities as they adapt to the new realm of data management in clinical trials, including benefits associated with risk-based quality management methodologies guided by RBQM/ICH E6 recommendations.
What is Meant by the “Future of Data Management?"
Traditional methods of data acquisition and clinical data management have been disrupted in ways that have forced regulators and the industry as a whole to advance their thinking on how to enable, and execute, new methods that can sustain the future of clinical development so that new medicines continue to be developed for our patients. While there has been amazing and rapid advancement in the development of technologies, such as the wide array of digital health technologies, for clinical trials, clinical data management processes have not kept on pace. Without modern tools, clinical data managers and data analytic groups have had to deal with an enormous and mounting burden to keep afloat in a sea of high-volume, high-velocity data.
The future of data management signifies that intelligent and automated tools are being deployed for data review and detection, with actionable analytics forming the backbone of the clinical data ecosystem while leveraging the benefits of risk-based quality management approaches and Quality by Design (QOD), as outlined in ICH E6 (R3) and ICH E8 (R1), respectively (the ICH website provides additional information on these and other relevant guidance). Modern clinical data management approaches use systematic, prioritized, and risk-based quality management approaches for clinical trial monitoring. Adapting to the modern clinical data management paradigm involves setting up informed central monitoring strategies to analyze the data coming in from electronic data capture (EDC) and other sources in almost real-time. To avoid post-database lock delays, it is important that sponsors and CROs proactively identify and mitigate any adverse trends in the data that can cause downstream issues for biostatistical analysis. To date, ICH GCP E6 (R3) has been adopted by multiple regulatory bodies and is key for quick study start up, ongoing data cleaning, and data analytics.
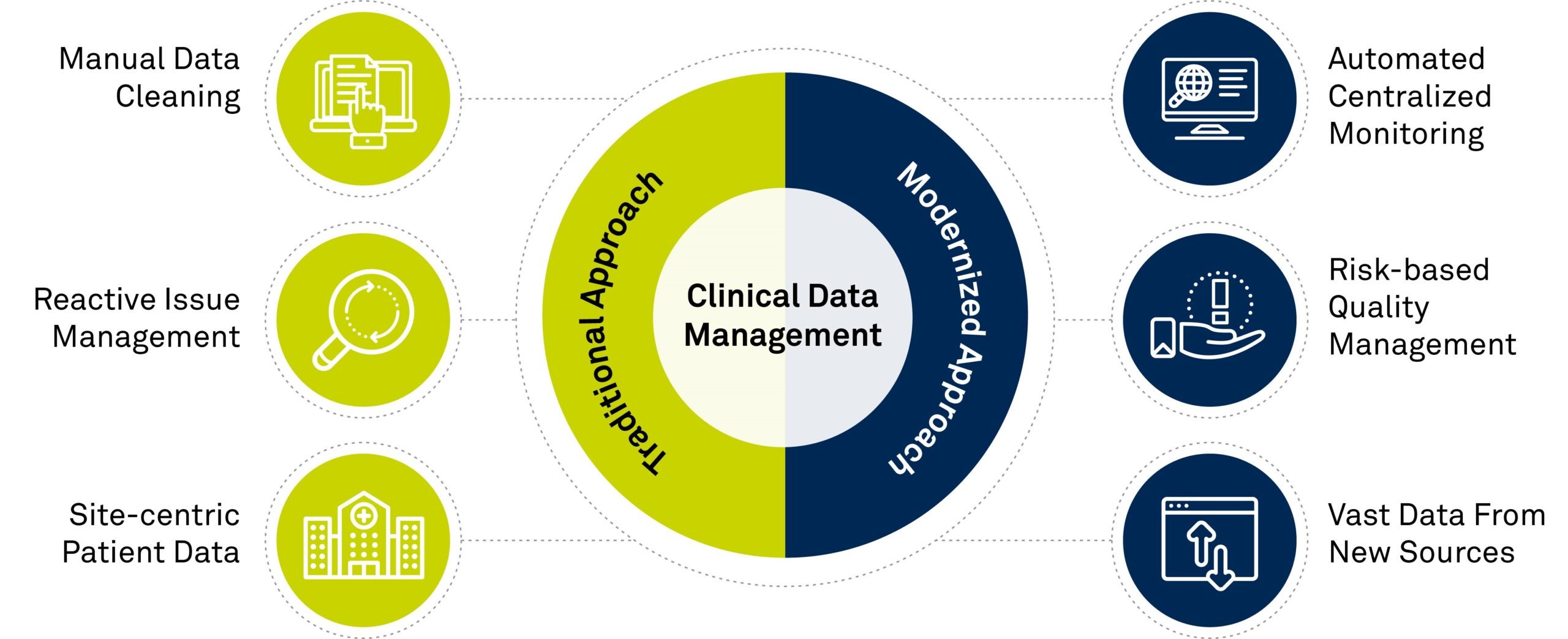
A Visual Representation of the Key Differences Between the Traditional Approach and Modernized Approach to Clinical Data Management
Organizational Impact of Risk-Based Quality Management
The clinical data management industry has traditionally been recognized as highly risk-averse and to stay with well-entrenched processes that often adhere to decades-old regulations.
Adopting the significant changes included in ICH E6 (R3) in a highly regulated atmosphere requires a thoughtful strategy, changes in management plans, and implementation that will require both process and cultural changes. For instance, COVID-19 has forcibly expedited organizations’ change from a process and cultural perspective, and it has brought forth the view that traditional clinical data management methods are not always scalable, and they are not adequate for supporting real-time review and analyses required by modern clinical trial designs that are becoming increasingly decentralized (see below for more on the impacts of decentralization on clinical data management).
A focused implementation team is key for enabling the optimization of ICH E6 (R3) since it can identify affected people, processes, and cultures and develop a long-term strategic approach. Some of the features for enabling the optimization of ICH E6 (R3) include the following:
- Agility—Study designs must enhance their focus on data acquisition.
- Common data model—Governance model must support a wide variety of data to ingest from an increasingly diverse group of sources.
- Patient-centric—Study designs should be “patient-centric” and always keep the patient in mind when making changes in data capture to meet patient, site, and study needs.
- Scalable—Data capture must allow for all types and volumes, ranging from labs to devices.
- Security—Data security is the custodian of patient trust.
Decentralized Trials—What Are Their Impacts on Clinical Data Management?
The arrival of the COVID-19 pandemic created a sudden urgency for alternative approaches to clinical trial execution and data capture given that trials experienced severe decreased enrollment or were halted altogether due to diminished patient ability or willingness to visit sites, delays in trial activities, and difficulties adhering to protocols. This situation forcibly expedited the shift to using decentralized methods for many trials which allowed them to continue while demonstrating the industry’s ability to quickly adapt to this new environment. While many CROs and sponsors had certainly explored new ways in which they could reinvent the clinical development paradigm, these were mostly viewed as one-off attempts or siloed efforts within an organization. However, in retrospect, these efforts were important in that they enhanced industry learning, and helped to lower concerns associated with potential risks, and increased confidence associated with alternative ways to conduct a clinical trial. In short, these pioneering efforts were the keystone of our learnings associated with remote data collection, data capture, and decentralization.
What is a Decentralized Clinical Trial?
In its simplest form, the term “decentralization” is defined as follows:
“Transfer (control of an activity or organization) to several local offices or authorities rather than one single one.”
If this basic definition is applied to clinical trials, it translates to transferring one or more capabilities or activities from how/where it is currently conducted to a different place. By applying this simple concept in combination with advances in technology, patients are now empowered to participate in clinical research across a variety of different environments.
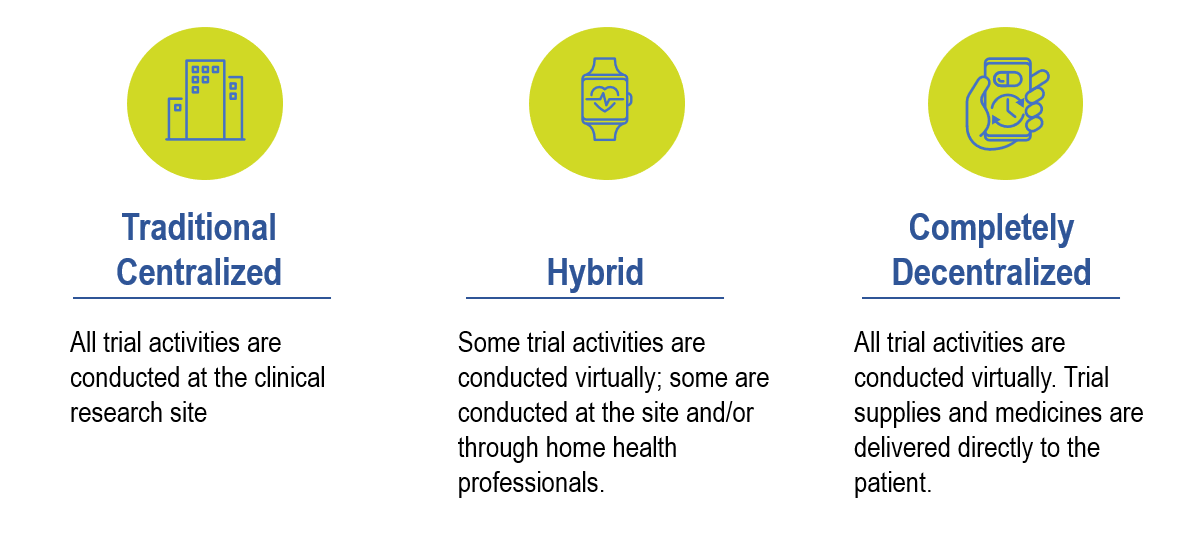
Given protocol complexities and uniqueness, it is difficult to apply a one-size-fits-all decentralization model to every clinical trial design. Therefore, the level of decentralization needs to be taken into consideration on a per protocol basis and the risks defined upfront. The following image depicts the different degrees of decentralization from Fully Decentralized to Fully Centralized:
At the ACRP 2021 virtual conference, an FDA official noted several “actual and/or perceived benefits” of DCTs:
- More efficient clinical trials at lower costs
- Accelerated enrollment and increased diversity
- More frequent measurements
- Reduced time and travel burdens for patients
Some of the key technologies that are allowing for the rapid adoption of decentralization include the following:
Patient-Facing Technologies are enhancing the patient experience and increasing study retention by providing, among other factors, easy access to information, user-friendly training materials, trial updates and alerts, and telehealth visits.
Protocol Development and Design Capabilities allow for a greater understanding of site and patient burden during the protocol development phase. This is key for developing a clinical design with the appropriate amount of ‘decentralization’ within a trial while maintaining the overall probability of success of the trial.
Remote or Onsite Consent Capabilities are providing flexible and consistent information exchange and data capture, while maintaining regulatory compliance.
Outcome Data Capture Technologies are rapidly being adopted in clinical trials (eCOA, wearable devices and sensors, apps, etc.), which is a trend that is expected to continue and accelerate. The rationale, purpose, and goals for including digital technologies in a trial can help inform what the best tools will be for the job at hand. Data interoperability, ingestion, and access are all important technology factors that should be taken into consideration as early as possible.
Data Oversight in a decentralized trial can often differ from a traditional “centralized” clinical trial. This is because critical data elements and data sources may vary from the norm, such as data being streamed directly from a sensor/wearable. Regardless of how/where data collection occurs, data platforms that allow for real-time/remote access of all clinical trial data collected is key in the modern clinical trial environment. Further, the role and skills of clinical data managers may also look different, and clearly defining the strategy, roles, and responsibilities associated with data oversight are critical first steps.
Issue Management can now occur in near real-time given that issues related to data anomalies, quality, and gaps can be identified and resolved in real-time. Technology enablers that allow for quick issue management allow for enhanced overall data quality, efficient and effective processes, and an automatic audit trail for transparency.
DCT Benefits to Patients
Since decentralized clinical trials are patient-centric designs by nature, they have multiple tangible benefits for patients, ultimately resulting in better recruitment and retention rates. By incorporating DCT-enabling technologies from beginning to the end of the clinical trial, patients can be offered a lower burden trial experience with more flexibility in logistics (e.g., direct-to-patient drug supply) and reduced time and travel burden. Further, sites and sponsors also have a better experience which together bolsters a clinical trial’s overall probability of success.
DCT Impacts on Data Management
Clinical trials are currently being modernized to include more decentralized elements. This increasingly digital future means that huge data volumes—ranging from simple laboratory test results, smartphones, and web apps to high-volume, high-velocity data from medical-grade sensors—will require proactive, automated, and intelligent risk-based quality management solutions for clinical data management. The traditional and outdated manual processes are quickly becoming insufficient for dealing with modern trial designs. Today’s clinical data manager must proactively plan and consider interoperable technology-based solutions to replace query and listing-based reviews of data. The role of the clinical data manager plays an integral part in combining disparate data to clearly communicate a patient’s journey.
Clinical data management organizations need to prioritize upskilling clinical data managers and dedicate time and resources to expand their analytical mindset, clinical skills, risk and mitigation processes, and understanding and use of real-world evidence, data trends, and new clinical endpoints based on alternative EDC designs from decentralized clinical trials.
Summary
The future of clinical data management has arrived, and it is scalable, flexible, intelligent, and interoperable with third-party tools. As sponsors and CROs increasingly turn to decentralized trial models, a thoughtful strategy must be established to provide the best opportunity for clinical trial success while reaping additional benefits such as increased efficiencies, cost-savings, security, and accessibility.
This is not the end! Stay tuned for additional white papers that will cover topics such as differences between decentralized clinical trials versus “virtual trials,” how the shifting clinical data management landscape is impacting data management (i.e., the impacts of direct-to-patient data capture on current processes), how to address new challenges associated with decentralized trials (e.g., reactive to proactive risk-based quality management processes, changes to skill sets required by clinical data managers), and statistical concept foundations and data visualization for effective use in data cleaning.
Check out Medidata’s unique Explorer Tool where you can select specific clinical data management challenges and view content related to those challenges. And don’t forget to download a copy of our white paper series focused on the future of data management to learn more about how clinical data management processes are being modernized to meet the mounting data pressures of today’s clinical trials.
Read our full blog series, titled The Future of Clinical Data Management.
Pillar Post: Is the Future of Clinical Data Management Finally Here? Indeed It Is!
Post 2: DCT Benefits to Patients and Impacts on Clinical Data Management
Post 3: Technology Enablers that Allow for Decentralized Clinical Trials
Post 4: Adapting to Modern Clinical Data Management Methods Requires Cultural Changes
Post 5: Adapting to Modern Clinical Data Management Methods Requires Process Changes
Explore Related Articles
Contact Us


