Building Patient Trust: Empowering Patients to Participate in Clinical Studies


This blog was authored by Paul Chang, SVP, Design and Experience at Medidata.
The decision to participate in a clinical study often comes at an emotional and vulnerable time for patients when more standard treatments have proven ineffective. Patients may have questions about the process—they may even have preconceived notions—and these notions can contribute to uncertainty and fear.
With big pharma at the bottom of the industry-positivity rankings, recruiters face an uphill battle convincing patients to join a study. While there are many factors that contribute to a successful recruiting campaign, building and nurturing patient trust plays an important role in smoothing the inherent speed bumps along the way.
But how do we identify trust-building opportunities among a group of patients who may have very different goals and perspectives? Statistics can provide indicators of high churn along the recruitment funnel, but the causes of drop out can only be identified by talking directly with patients.
In this article, we share a design process that we use to understand patients’ goals, expectations, and perceived risks, and ultimately to identify and prioritize trust-building opportunities.
Mapping Patient Trust
While trust itself is an emotional, social construct, our ability to build trust—or more appropriately, to avoid losing trust with patients—can be distilled to understanding their personal goals, expectations (particularly false expectations), and perceived risks.
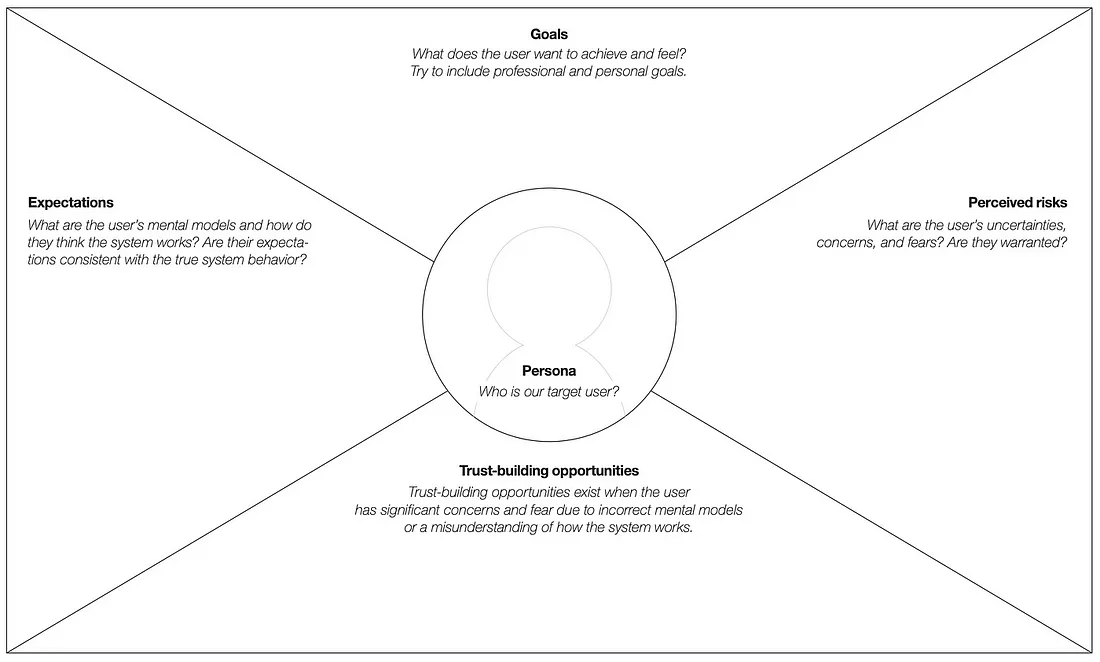
Knowing a patient’s personal goals helps us understand what motivates them to act. Goals also help to contextualize expectations and perceived risks.
False expectations are the result of a patient not understanding how a clinical study works or a predisposition based on previous experiences. For example, a patient may assume that frequent visits to the clinician’s office are required even though some studies may provide a more convenient means of participating from home.
Perceived risks, whether warranted or not, are equal in the mind of the patient. A patient considering a vaccine trial, for example, may fear becoming infected by the virus. For traditional vaccines, this risk may be warranted, while for newer vaccines that don’t incorporate the actual virus, the risk is perceived. In either case, the patient’s concern must be addressed if they are to move forward.
Trust-building opportunities arise at the intersection of false expectations and perceived risks. Consider the following trust-building priority space:
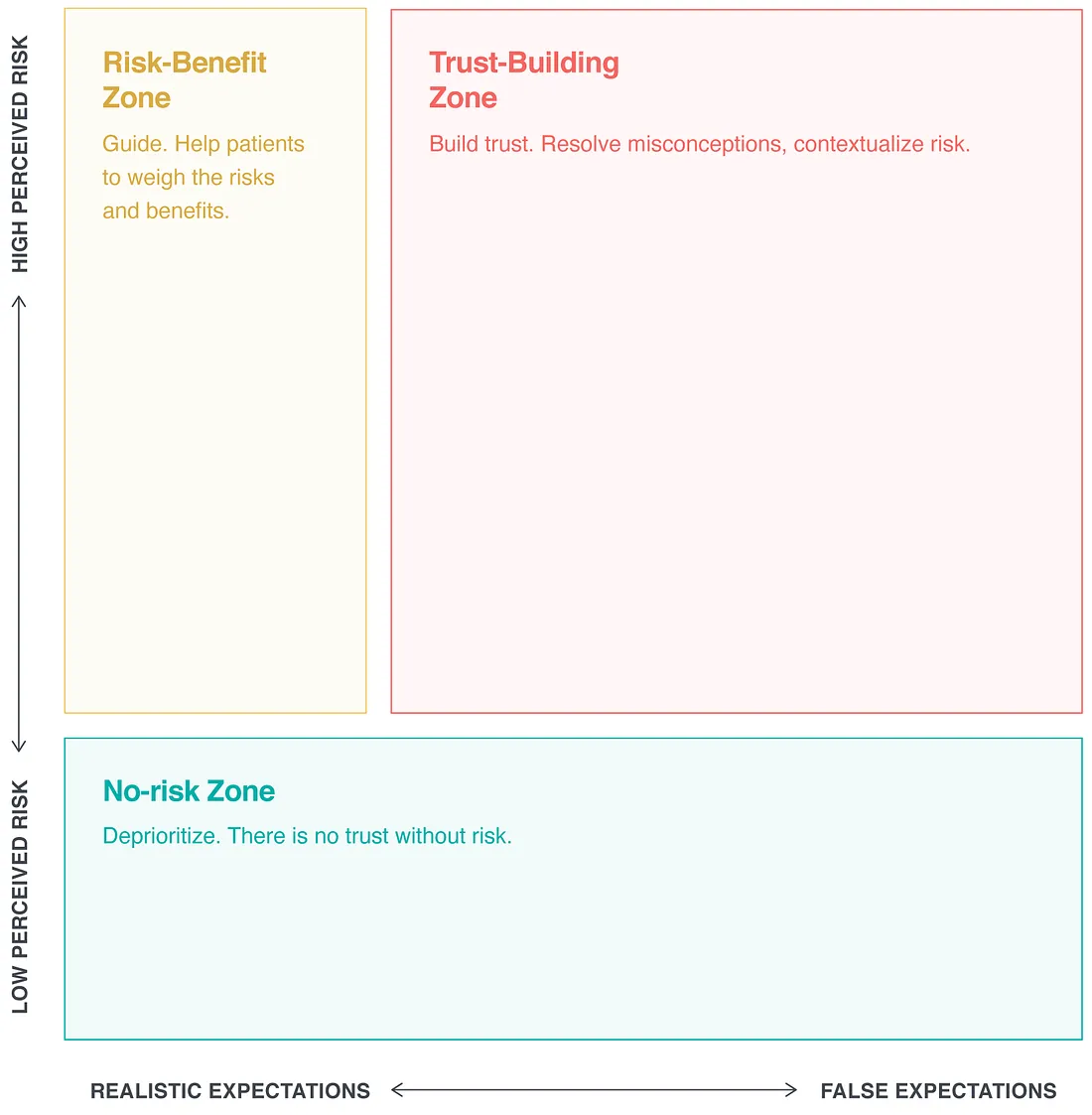
Without a perception of risk, trust is irrelevant. When expectations are realistic, trust is no longer an issue. In this case, patients must weigh risks and benefits.
An Example
We facilitated a workshop with five patient advocates to understand trust factors that could influence a patient’s decision to join a clinical study. We asked the following questions:
- What do you hope to get out of participation? How do you want to feel along the way?
- What are your expectations and how do you think the process works?
- What are your uncertainties, concerns, and fears?
We then identified emergent themes and derived trust-building opportunities.
The results were summarized in a trust-empathy map:
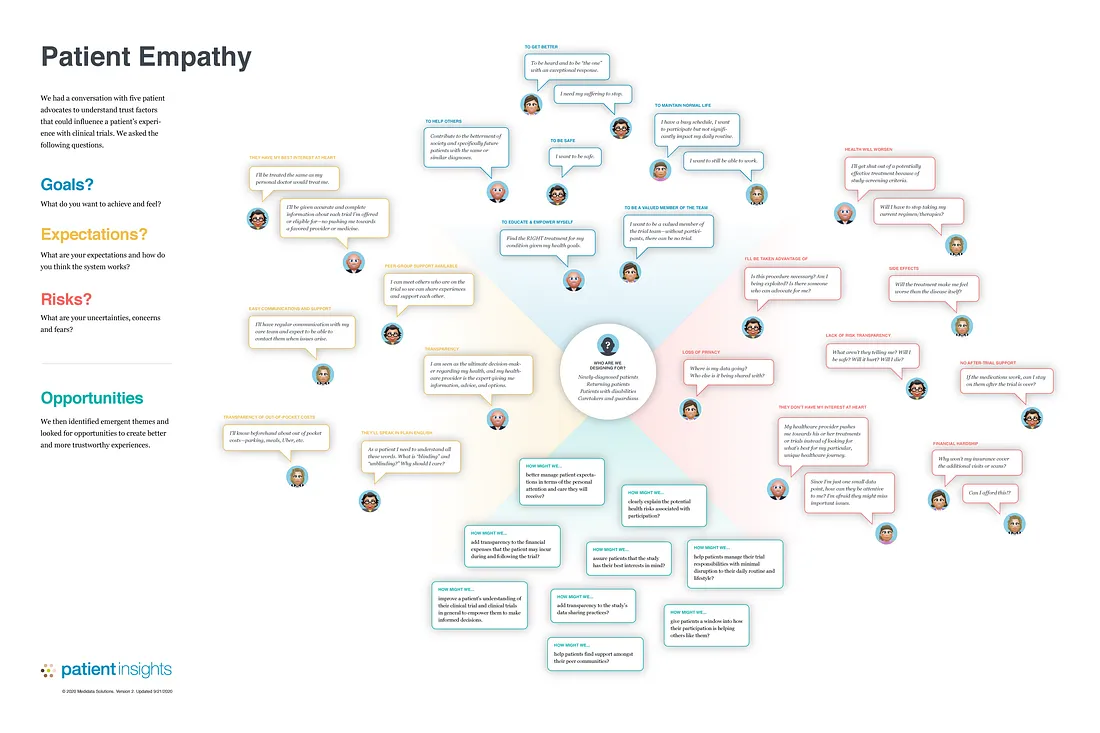
Trust Empathy Map inspired by Dave Gray’s original empathy map (detailed view).
The trust-building opportunities identified and prioritized were:
- How might we add transparency to the study’s data sharing practices?
- How might we clearly explain the potential health risks associated with participation?
- How might we assure patients that the study has their best interests in mind?
- How might we better manage patient expectations in terms of the personal attention and care they will receive?
- How might we add transparency to the financial expenses that the patient may incur during and following the trial
- How might we help patients find support among their peer communities?
Summary
Participating in a clinical trial is a commitment with associated risks and benefits. Additionally, patients may deliberate with perceived risks based on their understanding of the process and business of clinical trials.
In this article, we introduced a systematic approach to identifying trust-building opportunities with potential clinical-trial patients:
- Understand patients’ goals, expectations, and risks
- Derive and prioritize trust-building opportunities based on perceived risks and false expectations in the context of personal goals
With a research-backed, prioritized list of trust-building opportunities, we can now move confidently to ideation with the goal of recruiting more educated and empowered patients who in turn will be more likely to make an informed decision. Those who decide to participate are more likely to complete their study.
Contact Us
