Committed to Patient Data Privacy in Clinical Trials for Life Sciences Partners

For more than 20 years, Medidata’s customers have trusted us with their most valuable commodity: patient data. It's an honor and responsibility we take seriously.
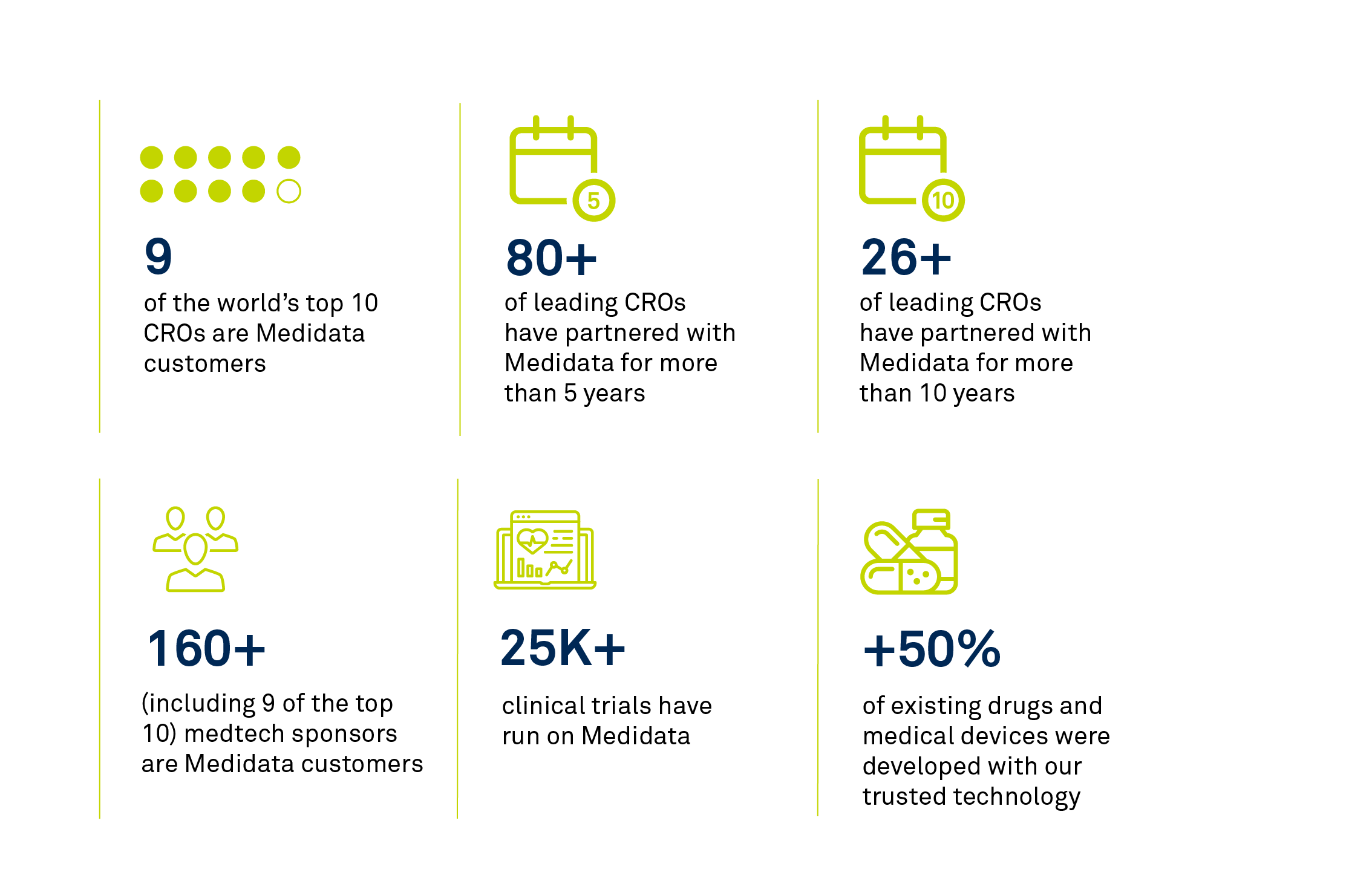
Patients and trial participants trust sponsors to collect, analyze, and secure data responsibly. And all over the world, those sponsors trust Medidata to ensure data privacy in their clinical trials.
From Global CROs and emerging and mid-sized biopharma organizations, to large biopharma and medical device companies, leaders of the life sciences industry continue to trust their reputations to Medidata.
“What differentiates Medidata is that you understand data. You are not only a technology company, but you are invested in high-quality data to meet our primary, efficacy, and other safety endpoints.”
– Prasann Mehta, Director, IRT & COA Services, Merck & Co.
Our Unified Protection Strategy—which unites our information security, data privacy, and quality teams—provides the combined security and quality required to achieve success in a clinical trial execution.
In-House Expertise Builds External Confidence
Avance Clinical is Australia’s largest specialist CRO, with a strong focus on international biotechs. Their clients trust them to provide fast and adaptive solutions in the early stages of drug development. And Avance Clinical relies on Medidata to build and nurture that trust.
“Avance Clinical understands that ultimately, it's all about the data. Medidata is our data platform that enables us to deliver powerful quality data, AI-powered insights, and patient-centric solutions.”
– Yvonne Lungershausen, CEO, Avance Clinical
Avance Clinical has developed a large team of accredited Medidata experts in-house, which not only demonstrates a commitment to being on the forefront of innovative technology, but also underscores that patient data privacy and management is a top priority for the company.
In fact, having this in-house, specialist expertise has been a deciding factor in winning contracts from many of the international biotechs they partner with.
“Medidata is a trusted and world-class offering, which means our clients are reassured they are working with a CRO that invests heavily in industry-leading technology and highly-trained, in-house operators,” Lungershausen says. “We are strongly supported by the Medidata team through their partner program, which means the highest level of expertise for our biotech clients.”
Supporting the World’s Most Vulnerable Patients During a Global Crisis
Clinipace is a global, full-service CRO that prides itself on being a collaborative partner with clients and ensuring clinical trial transparency.
When the global COVID-19 pandemic upended their clinical trial process, they relied on their trusted partner to help them adapt quickly.
“The last thing that anyone wanted to do in 2020 was stop clinical research. It's just too important to patients who are looking for solutions. We needed to make sure we could support those trials and that we could deliver quality data at the end of the day.”
– Lindsay Talley, Vice President, Global Clinical Data Management, Clinipace
During the pandemic, Clinipace and Medidata worked very closely to find innovative ways to continue their important work, despite the new challenges they faced. It wasn’t easy, but ultimately, it was successful.
“We brought many ideas to the table. ‘Maybe we can try this,’ or ‘perhaps we could do that,’” recalls Talley. “It was a very disruptive time, which is why it was so wonderful to have experienced partners like Medidata that were honest and transparent with us. We heard things like, ‘Absolutely we can do that,’ or, ‘that won’t work, but here's a different way we may be able to achieve what you're looking for.’"
Due to the collaborative and creative nature of the partnership, Clinipace was able to keep their EDC running properly and ensure that any randomization activities or modules needed to meet data collection requirements were completed.
“Thanks to Medidata, we were able to help each of our customers bring their compounds forward to achieve their primary and secondary objectives, even in a very difficult time,” says Talley.
De-Risking Clinical Trials Through Better Data Management
Medicenna Therapeutics is a clinical stage immunotherapy company, tackling some of the most challenging—and rarest—diseases.
Due to the rarity of the conditions Medicenna works with, they can face unique challenges in their clinical trials, occasionally struggling to collect enough patient data and published literature to move through the phases.
But with Medidata technology, they were able to modify a control arm within a Phase II trial, and glean keen insights about a test drug’s efficacy and safety.
“Medidata has this incredible ability to generate a Synthetic Control Arm, which allowed us to be less dependent on literature data. Using their know-how and expertise, we were able to collect data from patients that had been matched nearly equivalent to the patients treated in our clinical trial.”
– Fahar Merchant, PhD, CEO and President, Medicenna Therapeutics
The process allowed Medicenna to substantially “de-risk” what might normally occur in a Phase III clinical trial, ensuring patients in the treatment arm trial were nearly identical to those that would have received the standard of care. It generated a data set that gave them the confidence to approach the FDA to request clearance for a Phase III trial.
“We showed them the example of what Medidata had done for us in a Phase II trial and leveraged that information to get further approvals for the very first design of a Phase III registration trial, where the majority of patients would come from a synthetic control arm or an external control arm,” says Merchant. “We are a smaller company, so that was a big accomplishment for us. When Medidata talks about solving the impossible, I believe it, because the impossible is basically what we were able to accomplish.”
Explore Related Articles
Contact Us