Decentralized Clinical Trial Data Flow Maps | Association of Clinical Research Organizations

This blog was authored by Fiona Maini, Global Compliance and Strategy Principal, and Valeria Orlova, Global Compliance and Strategy Analyst.
As decentralized clinical trials (DCTs) are becoming increasingly more abundant, there is still a need for higher transparency in the industry concerning the digital format of clinical data. From the traditionally paper-based conduct of clinical trials, to the new integration of data-capturing devices and applications, it is not surprising that the new and updated system for data transfer of a DCT needs to be clarified and accentuated.
The importance of data transparency was noted in the joint symposium by the MHRA and FDA on the Considerations for the Design and Conduct of Decentralized Clinical Trials: Regulatory Perspectives. In particular, the regulators highlighted recommendations for data collection, handling, and management, including data flow maps for sponsors and CROs to demonstrate data transfer, collection, and processing.
The Association of Clinical Research Organizations (ACRO) DCT Working Party, chaired by Medidata’s Fiona Maini, set out a goal of building transparency and visibility by creating a set of data flow maps for five components of a DCT. These components include eConsent, direct-to-patient supply of the investigational product (IP), investigator-participant interactions, connected devices, and home health visits. The data flow maps provide a general, industry-wide overview of the typical data flow environments. This may serve as a starting point for building data flow maps, enabling complete transparency of how data is captured and flows, and showcasing that data integrity is ensured to a high standard through decentralized clinical trials.
There are multiple DCT components, and most importantly, many possible combinations of these components that exist due to the flexibility of a decentralized or virtual approach (which can be both hybrid and completely remote). The specific data flow configurations will vary significantly based on the trial design, the DCT components, and the data systems integrated into the trial, as well as the variation between standard procedures for data handling across third party vendors.
The below data flow map is a preview of the five maps available on the ACRO website for direct-to-patient supply of IP. This overview demonstrates how the data is transferred and synchronised between data systems in the end-to-end process of delivering the IP to the participant’s location—either directly to the participant’s home, to a local site or pharmacy, or via a home healthcare nurse visit.
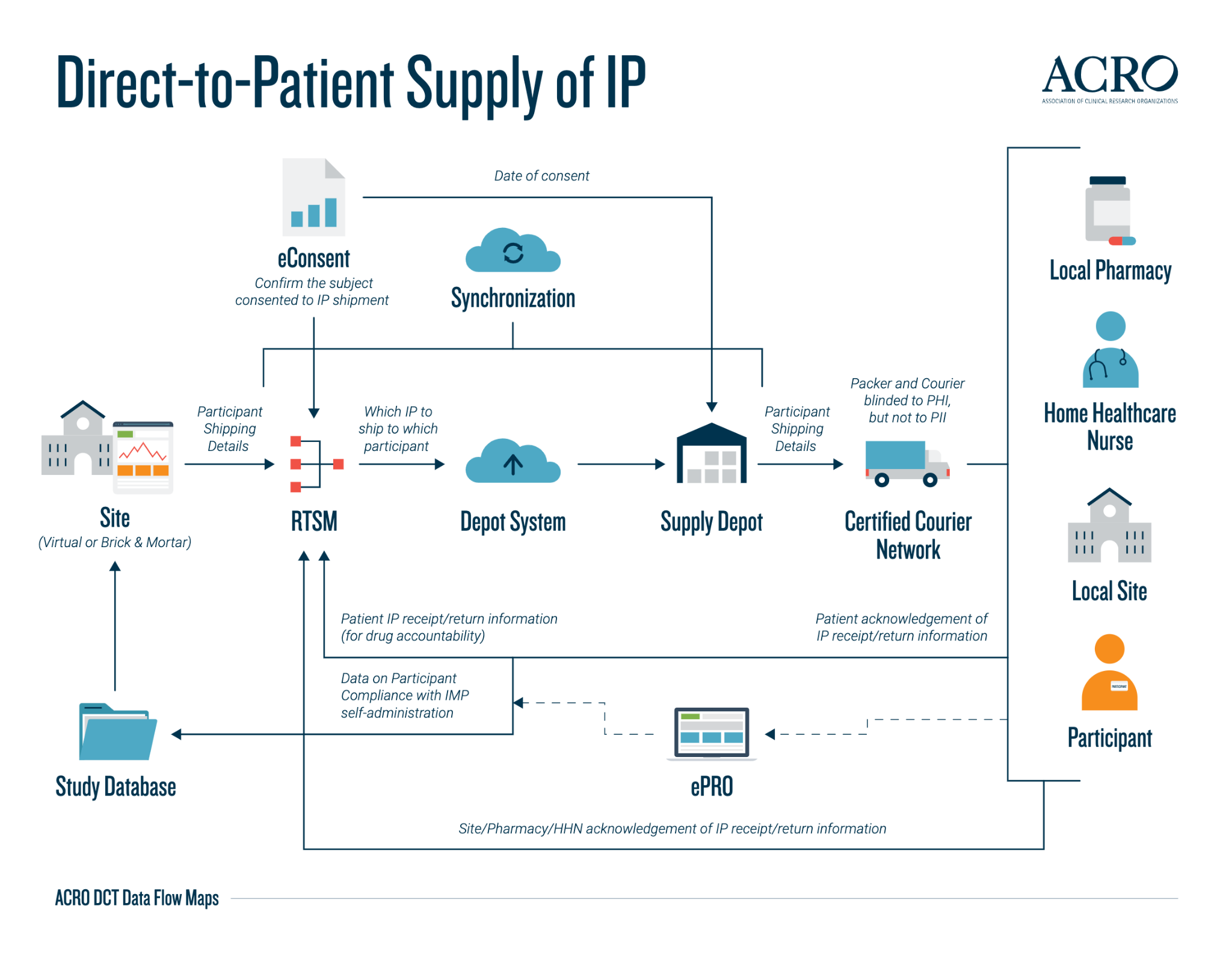
The data flow maps provide a clear and visual insight into the commonly used data systems, the type of data stored in the system, and some visibility into data processing, as well as a complete overview of the typical journey of the data transfers that may occur in a DCT (along with any potential variability). The data flow maps serve as an addition to the existing and expanding ACRO DCT Toolkit—a collection of materials produced by the Working Party on implementation considerations for decentralized clinical trials. This toolkit is dynamically evolving with Medidata’s continuous inputs.
The ACRO Data Flow Maps are a first-of-a-kind generic tool that provides insights into the dataflow of decentralized clinical trials. Medidata is proud to have been a key contributor to this project.
Contact Us
