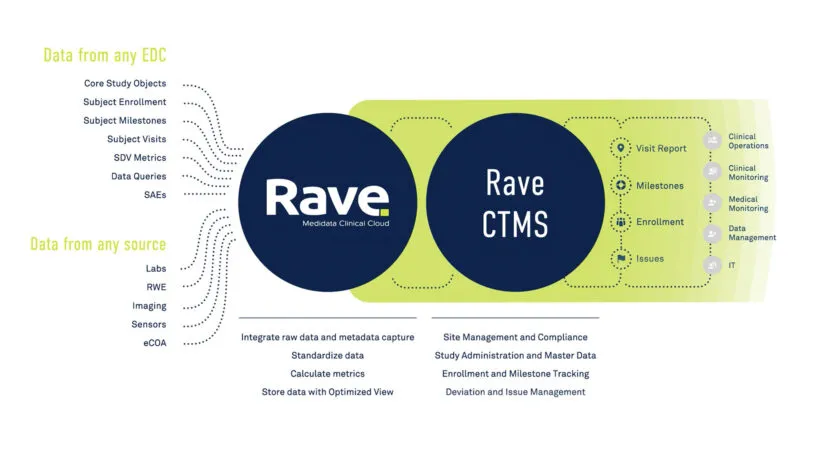
Real-time Clinical Trial Analytics: Medidata Intelligent Trials
Increase confidence in decision making across a clinical trial’s life cycle – from study planning through execution – to bring treatments to patients faster. With our turnkey solution that provides unmatched site-level granularity and actionable real-time industry-wide insights, you can reduce trial timelines, prevent enrollment delays, enroll more diverse patients, and ensure success of your clinical program.
Benefits of Clinical Trial Analytics
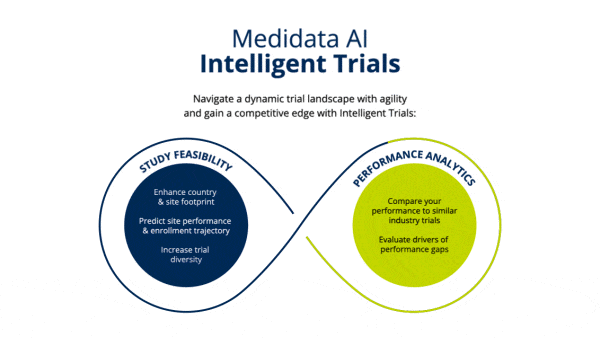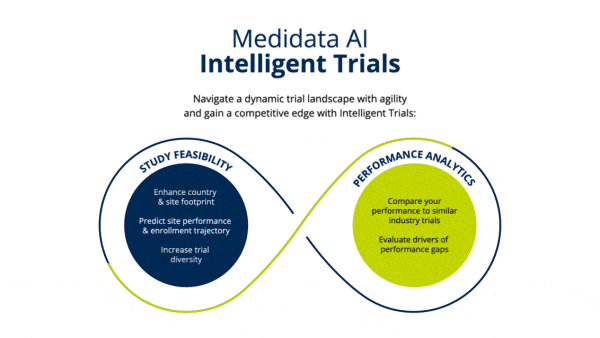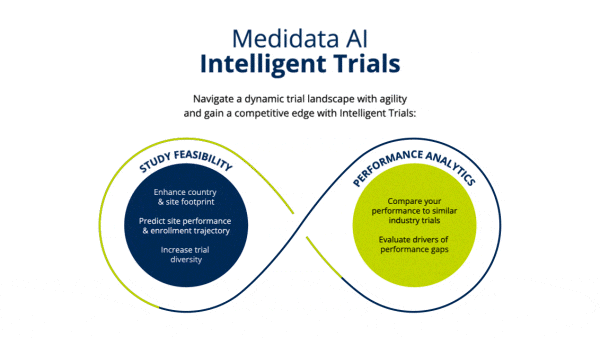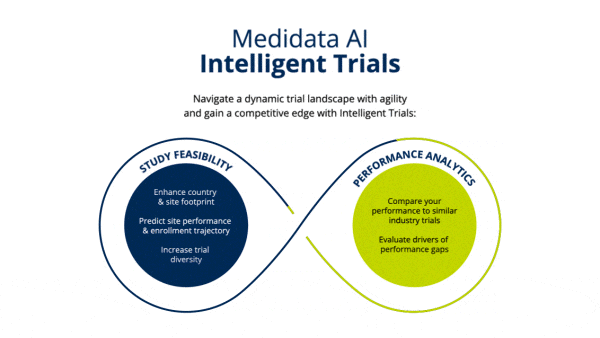
Identify Study Footprint
Determine realistic timelines and study footprint by assessing enrollment rates of industry trials and selecting which countries and number of sites would be needed to meet timeline goals.
Learn more about Intelligent Trials Study Feasibility.

Select High-Performing Sites
Improve site selection to meet enrollment timelines leveraging industry-wide, real-time and historical enrollment performance and predictive analytics.
Learn more about Intelligent Trials Study Feasibility.

Enroll More Diverse Patients
Establish clinical trial diversity goals and enhance site selection to choose sites that can accelerate your trial and are more likely to enroll diverse patients, based on indication-specific cross-industry data.
Learn more about the Intelligent Trials Study Feasibility Diversity Module.

Accelerate Enrollment
Identify opportunities to improve operational performance and accelerate enrollment by comparing your trial to the performance of industry trials and forecasting enrollment.
Learn more about Intelligent Trials Performance Analytics.
Key Features

Industry’s Largest Performance Dataset
Cross-pharma and CRO dataset that spans over 33k trials and 10M patients, adding unique value to even our largest customers.

Unmatched Site-Level Granularity
Metrics span operational and diversity performance, enabling selection of sites that will accelerate the study and increase clinical trial diversity.

Actionable Real-Time Insights
Unique insights leveraging data refreshed weekly from ~8k active studies, allowing customers to calibrate performance and take action within a competitive landscape.

Turnkey with White-Glove Support
Off the shelf SaaS solution and API access combined with white glove service support customers in making confident decisions.
Learn More

Featured Whitepapers and Reports
The State of Black Participation in Clinical Trials
In our new research report, we set forth to assess the current state of racial diversity beyond the FDA Snapshot, using Medidata’s industry-leading clinical trial data repository.

Featured Case Studies
Medidata AI Supports PPD’s $60M Study Award in Rare Oncology Indication
With Medidata’s broad industry performance data and Study Feasibility solution, PPD was enabled to reduce the projected clinical trial timeline and be awarded a key $60M study in a rare oncology indication.

Featured Podcasts and Webinars
ASCO Education: Racial Disparities in Clinical Trial Participation
Learn about the state of clinical trial diversity in clinical trials and what clinicians and trial sponsors can do to improve participant diversity.

Featured Blogs
How Pharmaceutical Companies Can Achieve Success Amidst Regulatory Focus
As new rules go into effect, learn about the top things pharmaceutical companies should consider to increase diversity in their clinical trial planning and execution, including enhancing site selection.