Complex Trials – Innovative Trial Design and Emerging Approaches
Trial complexity is not going away. What can be done to ease the burden?
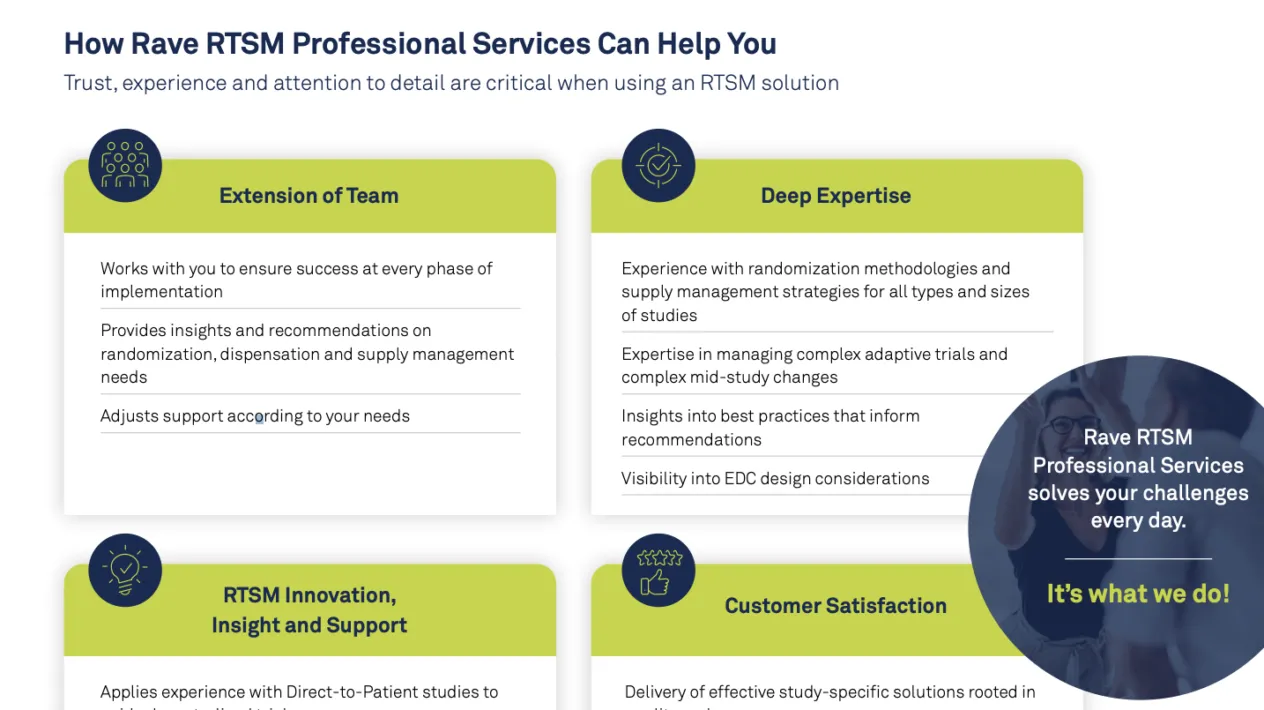
Medidata works with sponsors and CROs to simplify the complexity where possible, including:
- Consolidating and simplifying systems
- Reducing the patient burden
- Minimizing the site burden
- Accelerating data integration, standardization, and review
- Partnering with one experienced team that provides insight and support
What Customers Have to Say
Medidata Streamlines Complex Data Management
“A complex trial design and protocol means a complex database, which is incredibly resource intensive – whether that is site staff entering data, data managers or CRAs. Anything you can do to streamline is critical because resources are so precious.”
– Stephen Nabarro, Director of Clinical Operations, CRUK

Medidata is ahead of the game with complex trials
“Clinical trials are very complex, and when we are putting some of these systems together, and putting some of the forms together, it is extremely difficult. Medidata is keeping on top of that and keeping ahead of the game.”
– Ian Pinto, Chair, ACDM

Complicated logistics studies become less complex with Rave RTSM
“We use Rave EDC and we do a lot of work with Rave RTSM on complicated studies, which offer different ways of ensuring drugs to the right patients at the right time.”
– Earl Selzer, Partnerships and Innovation Lead, CTI

The Medidata Platform is enabling us to do more complex trials safely
“We’re a specialist provider, so we do a lot of complex studies. When you talk about accelerating the submission of platform studies, that’s interesting for us because that’s a way of being able to submit in multiple countries at one time. Complexity isn’t always about the trial design – it is getting the sequence right and doing the right thing. The Medidata Platform is enabling us to do more complex trials safely.”
– Andrew MacGarvey, COO, Phastar

Key Capabilities
The Clinical Trial Industry has worked tirelessly to reduce burdens across the ecosystem through technology, processes, and attempts at standardization. Despite this, complexity has continued to increase year-on-year. With the number of studies increasing annually, what are driving these increases?
Complex trial design results in an increased number of endpoints and higher volumes of data and analysis. Challenges increase exponentially across the spectrum of data management processes, including the collection of excess data, isolating gaps, managing duplication, integration with systems, and interoperability.
Traditional clinical trials have relied heavily on research sites acting as central locations, leading to long travel times to attend visits. This can be a barrier for patients looking to participate in life-saving trials. The decentralized trial model uses patient-facing technology to move trial activities outside central research sites and into patients’ daily lives. This shift lessens the burden of trial participation for both patients and sites, enhancing the overall clinical trial experience.
Inspired by Patients. Powered by Data. Securely Delivered to Scale.
Gain access to the most comprehensive decentralized clinical trial solution built from an ecosystem of tools, people, and processes that let patients, sites, and sponsors participate, contribute to, and monitor any clinical trials outside of a traditional investigative setting.
Reata Pharmaceuticals saves millions leveraging the adaptive functionality of Medidata Rave RTSM
Medidata Rave RTSM is a new generation of cloud-based RTSM capabilities include:
- Unparalleled agility and control for project sponsors and CROs
- New user paradigm-based on an accessible, 100 percent configurable interface
- Choice of deployment options; unified with Rave EDC or standalone solution
- Best of cloud-based, agile technology to streamline design and provide real-time visibility into operations
“We needed a system that accommodated our needs and could be easily modified during the trial. We found that system in Medidata. Their randomization and trial supply functionality present a unified solution with Rave EDC, so everything works together seamlessly. Rave RTSM is a ‘best-in-class’ solution that did not financially constrain us.”
– Sr. Director Clinical Study Manager, Reata Pharmaceuticals

Complex Trials – the Ever Changing Face of Complexity
When it comes to operational complexity and the multiple layers of clinical trials, Medidata provides way to conquer:
- Planning and forecasting global studies
- Adoption and implementation of decentralized clinical trials
- Integration of disparate systems
Benefits of Rave RTSM in a Complex Trial
Rave RTSM
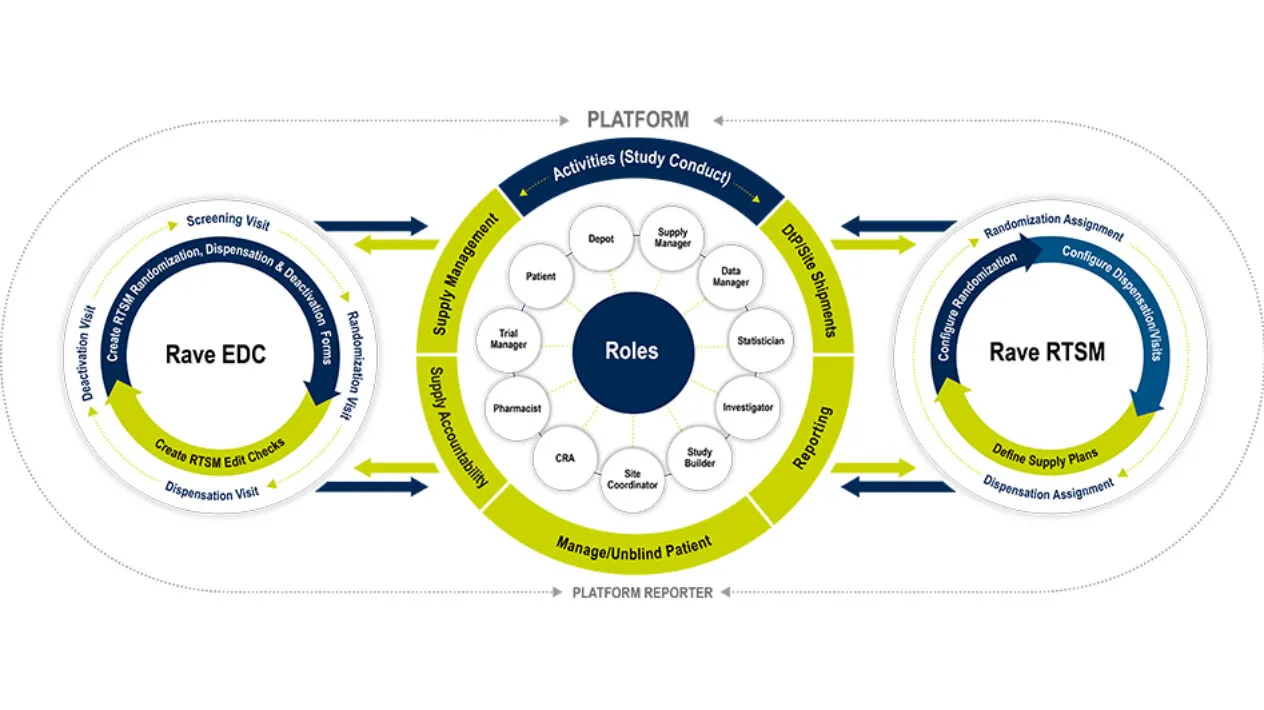
Rave RTSM is the only fully pre-validated randomization and trial supply management solution that can be configured in minutes and enables mid-study changes with minimal downtime and change orders.
RTSM is built on Rave EDC, so there is no double data entry and minimal reconciliation expediting study start-up and study-close out. Rave RTSM streamlines your operations and provides real-time visibility for your study teams.

Optimizing Direct-to-Patient Supply Management
Learn how CRO ClinChoice and the sponsor 89Bio were conducting a phase II, randomized, double-blind, placebo-controlled study to explore the efficacy and safety of investigational drug X in subjects with severe hypertriglyceridemia (SHTG).
Unification of Rave RTSM and Rave EDC
Rave RTSM is fully unified with Rave EDC with no customized integrations, no reconciliation required and is fully configurable and pre-validated. The Rave RTSM and EDC solution is a paradigm shift from IRT/EDC as separate vendor systems. Sites record patient visits in Rave EDC as Rave RTSM assigns randomization arms and inventory items in real time. There is no need for double entry which increases the overall data integrity and reduces risk.
Abbreviated Timelines
Rave RTSM can be configured and implemented in as little as 2 weeks. Its pre-validated capabilities enable a quick UAT with reduced risk.
For mid-study changes, study builders can modify the study using RTSM’s state of the art Edit Live Design which enables changes to occur in real-time without stopping enrollment.
Shorten the Duration from LPI to Database Lock
Rave RTSM and Rave EDC are completely unified meaning there are no customized integrations reducing risk and timelines.
Data between RTSM and EDC requires no reconciliation as is required for a separate IRT and EDC model. This enables studies to lock much quicker, resulting in considerable cost savings.
Reduction of Risk
Rave RTSM is configurable and pre-validated. This means that RTSM works the same for all studies. What differs from one study to the next are the configuration settings of Randomization, Dispensation and Supply Management. With RTSM, User Acceptance Testing (UAT) is abbreviated with a smaller percentage of findings.
RTSM solution’s reduced risk is the biggest differentiator against all other IRT vendors.