Large Biopharma: Clinical Trial Solutions
Adapt, innovate, and scale for the future
Global pharma and biopharma companies like yours partner with Medidata to accelerate timelines, streamline processes, drive efficiencies and stay at the forefront of the industry.
Why Global Industry Leaders Trust Medidata
You and your team are under pressure to continually innovate, adapt to disruptions, focus on patient centricity, detect risks earlier, and scale at an unprecedented pace to get therapies to market faster.
With 20+ years of industry leadership, Medidata empowers 19 of the top 20 biopharma companies to meet the demands and opportunities of every trial environment.
Experience Matters
With over 2 decades of worldwide collaboration with customers, patients, and partners, Medidata delivers smarter treatments and healthier people.
Clinical Trials
Thousands of studies in 140+ countries have been conducted on the Medidata platform.
Patients
Over 10 million patients across Medidata trials have impacted clinical research and touched countless lives.
of the top 20 pharma companies
95% of the top 20 pharmaceutical companies use Medidata technology.
Of FDA-approved Drugs
More than 7 out of 10 novel drugs approved by the FDA in 2022 were developed on Medidata software.
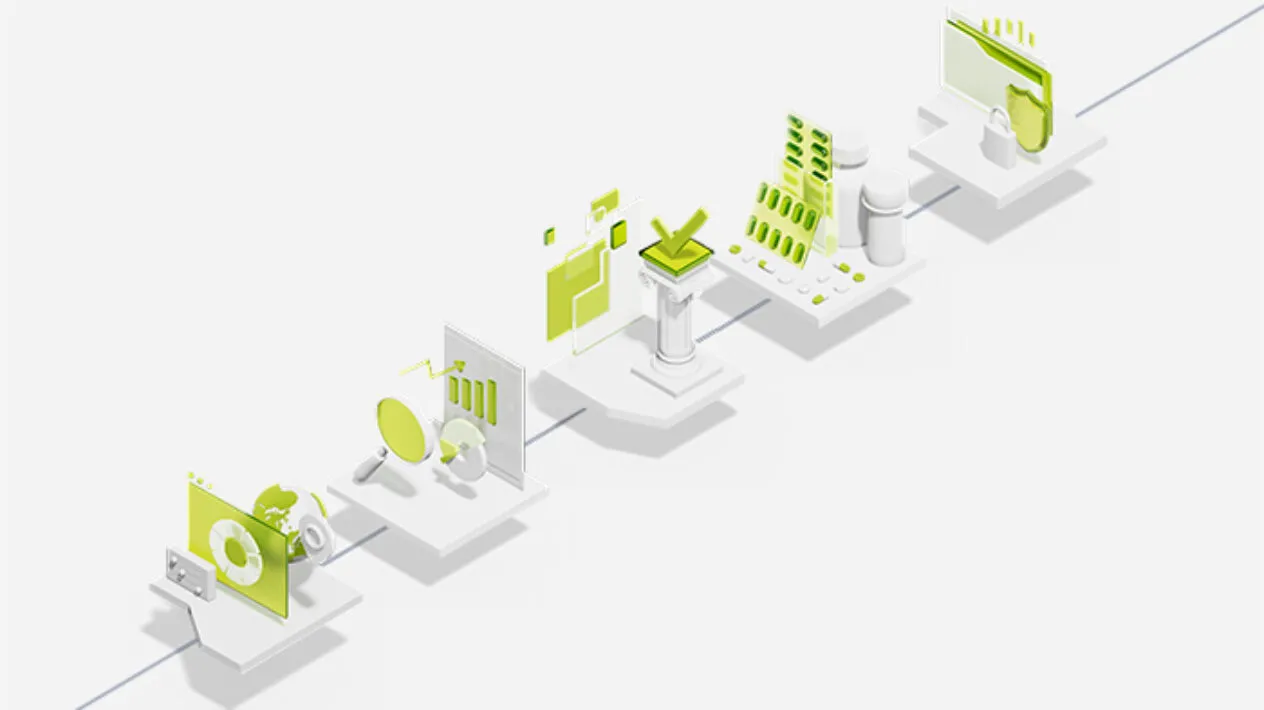
Your Technology Roadmap through the
Clinical Trial Process
Clinical trials are inherently complex and challenging to navigate and the right technology can help. Sponsors struggle with deciding which technology is right for their study requirements.
This clinical trial roadmap illustrates the technologies that add the most value at each step of a trial. No matter where you are in your trial process, design and planning, start-up, collecting and managing patient data, or closing out, step through the roadmap for guidance to bring your trial to a successful conclusion.
A Few of Our Partners & Customers

Medidata Signs Multi-Year Agreement with Bristol Myers Squibb to Extend Relationship and Continue Company’s Use of Platform
Collaboration underscores Bristol Myers Squibb’s commitment to unleashing the power of digital technologies to advance its mission to discover, develop, and deliver Innovative medicines that help patients prevail over serious diseases

Eisai Selects Medidata’s Clinical Data Studio to Enhance and Modernize Clinical Trial Efficiency and Patient Experience
Eisai Inc., will leverage this innovative data experience to gain unprecedented control over its clinical data, enable the execution of scalable and complex clinical trials, and enhance patient experience.

Global Pharma Bridges the Gap Between Data Management and Pharmacovigilance Site Count
A global life sciences organization engaged in an initiative to significantly improve clinical productivity. This industry leader typically has hundreds of new treatments in development at a time in all trial phases. The capture, triage and reporting of serious adverse events (SAEs) by local and central pharmacovigilance (PV) groups is a critical process that runs concurrently with data management.
A Top 10 Pharma Company Doubles High-Performing Site Count
A top 10 biopharmaceutical company was looking to gain agility and confidence in their trial planning, site selection, and enrollment decisions in a priority indication. The sponsor wanted to expand their site list and gain a deeper understanding of how existing sites in their database performed.
Resources

Find a Partner
Improve your performance with Medidata’s diverse ecosystem.