Power Innovation in Academic Clinical Trials
Our strong commitment to advancing academic clinical trials began over 25 years ago when Glen de Vries, a passionate researcher at Columbia University, co-founded Medidata. With more than two decades of experience, Medidata has established itself as a leader in supporting the complexity and nuances of your academic clinical trials.
We are committed to supporting your endeavor to enhance disease understanding and discover new treatment applications, aiming to drive meaningful breakthroughs in patient care. By leveraging our state-of-the-art clinical platform and expertise in setting up clinical trials, we provide targeted support to your academic institution, public entity, or non-profit foundation, enabling the achievement of important objectives.
Why Medidata for your academic clinical trials?
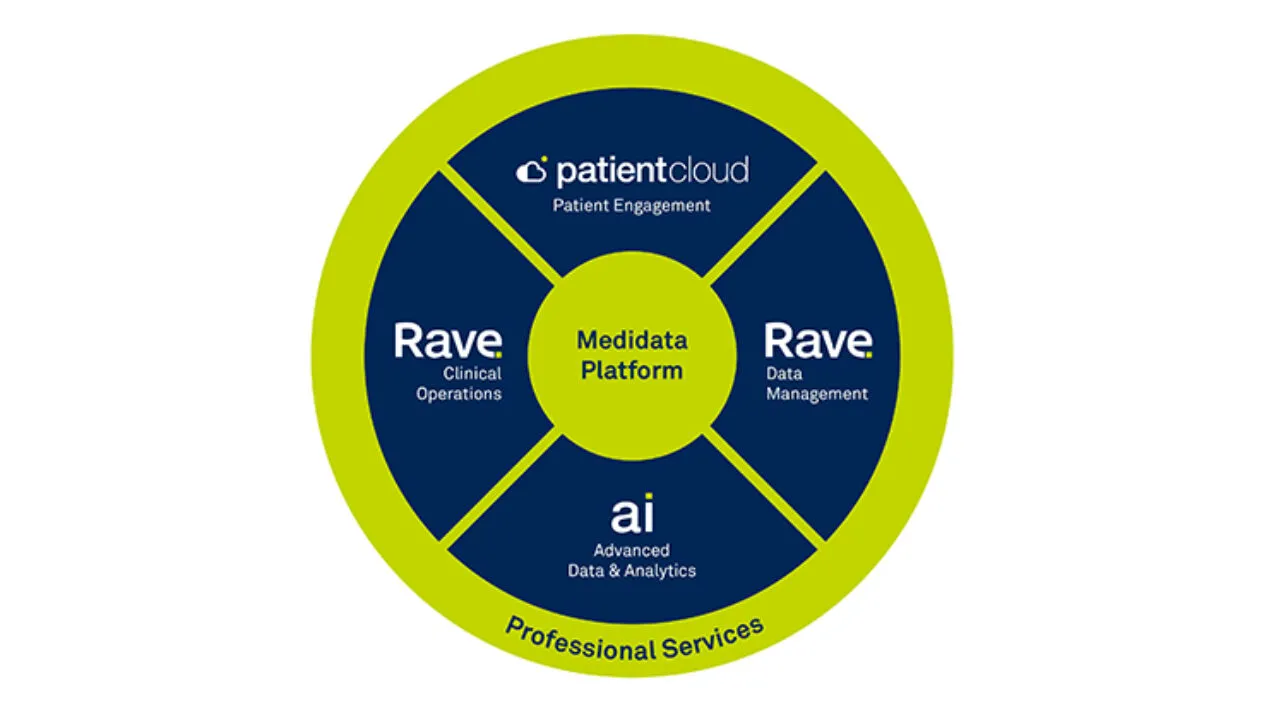
Power your entire clinical trial workflow on Medidata’s Unified Platform.
Our unified, cloud-based platform helps you cut development costs, mitigate risks, and power innovative breakthroughs faster.
Whether you need a secure EDC system, a pre-validated randomization and trial supply management solution, a streamlined electronic patient reported outcome and more, we provide a customized solution for your needs.
For academic organizations running clinical trials with increasing sensitivity in pricing and resourcing, Medidata makes it easy to complete builds using any of our suite of clinical trial solutions.
Our academic pricing model is designed to meet the needs and demands of academic clinical trials.
We recognize that academic clinical trials are often registrational or observational with little to no commercial incentive upon completion, therefore we have developed a pricing model to ensure universities, hospitals, government agencies and foundations can access Medidata technology to advance research.
An experienced team to support your trial needs.
Our academic enablement program is designed to empower researchers to build/configure their own studies as this is often more cost-effective than having Medidata or a third-party conduct this work.
With our award-winning professional services organization, you will not only learn how to configure your own studies, but also provide program-level support and mentorship upon completion so you always have a dedicated representative to support your ongoing concerns, needs, and questions.
Decrease patient burden to avoid recruitment and retention challenges.
Medidata’s eCOA solution with eConsent improves study start-up times and builds patient-rich experiences, accessible to patients through myMedidata.
Our eCOA is the industry’s only full-service solution supporting a truly integrated experience that easily & accurately captures data from patients, caregivers and clinicians, enabling patient diversity in traditional, hybrid or fully decentralized trials.
Improve Data Entry Quality and Reduce Time for your IITs.
Rave Companion and Health Record Connect reduce time spent in data entry, increase job satisfaction, reduce monitoring costs, and ultimately make for better, more efficient research.
This is done by streamlining the process of bringing data from your EHR over to Rave EDC.

Medidata Research Alliance
The Medidata Research Alliance is a strategic partnership initiative that links academic and medical communities with Medidata’s expertise in data and technology to enhance scientific research and patient outcomes. The alliance has effectively explored immunotherapies and rare diseases, with results presented at global conferences such as ASCO, ASH, EHA-EBMT, and NCCN. This model has proven successful and is being expanded to cover more therapeutic areas.
Specialists for Organizations Running Academic Clinical Trials
Optimize Medical Research and Improve Patient Care.
Medidata partners with universities and hospitals engaging in clinical research. Our innovative solutions, dedicated support, and tailored pricing model enable hospitals and universities to run investigator-initiated trials at scale on the Medidata platform.
Advance Your Cause With the Right Technology Partner.
Medidata partners with foundations for the trials they are involved with both directly and through clinical research organizations. We strive to understand the objective of the trials you are funding and help you select the best technology to achieve those outcomes.
Streamline your Technology Infrastructure to Conduct Research at Scale.
Medidata partners with Academic Research Organizations (ARO) by providing volumetric pricing, product enablement and dedicated program-level support and mentorship for the studies they are conducting on the behalf of academic sponsors. This allows AROs to select the right technology to successfully conduct trials on a unified platform for their sponsors.
Uniquely Positioned to Meet Security and Compliance Requirements of the Federal Government.
Medidata partners with federal governments to conduct clinical trials in both intramural and extramural settings. With over 20 years of experience supporting the federal government agencies, Medidata is up to date on the security and compliance requirements and has developed government-specific programs to maximize the success of government-sponsored trials.
We Understand Academic, Research, & Non-Profit Partnerships.
For over 25 years, we have served as a clinical trial technology partner for leading organizations across hospitals, universities, foundations, not for profit, and federal government segments.
Patients
We help you maximize patient engagement to drive better outcomes and better clinical trial results.
Sites
Sites trust Medidata to provide an excellent, easy-to-use platform that simplifies time-consuming administrative tasks.
Studies
We’ve helped leading researchers achieve major breakthroughs across thousands of studies and reaching hundreds of thousands of patients.
Sponsors
Research sponsors across the academic, non-profit, and commercial industries trust Medidata to deliver proven solutions for clinical trials.
Our customers trust us for proven results.

Duke Cancer Research Institute’s ADAPTABLE Study
“Compared with traditional cardiovascular trials that engage hundreds or thousands of sites, this technology allowed us to enroll 15,000 patients from 40 centers.”
–
Dr. Schuyler Jones
ADAPTABLE Co-Principal Investigator, Associate Professor of Medicine , Duke University Medical Center

Accelerating SCTU’s Digital Transformation
“Adopting Medidata’s platform has allowed for more people to engage with the data and for us to broaden the use of the data. We’ve shifted to the idea that everyone owns the data.”
–
Susannah Condie
Head of Clinical Data Management, Southampton Clinical Trials Unit

Frontier Science Keeps Trials Moving with Remote Source Review
“Speed and collaboration were our two project pillars. We quickly moved from idea to implementation by leveraging our strong Medidata working relationship. Their rapidity was striking; this type of speed requires a high level of dedication – where everyone on the team is committed to meeting an aggressive timeline.”
–
Marlene Coope
Frontier Science
Medidata is more than Rave EDC. We have the right sized solution for each step of your clinical trial.
Many academic institutions and researchers rely on Medidata’s best-in-class, proven technology to simplify, scale, and accelerate their clinical trials across all steps and phases (phase 0-4) of their studies—from protocol design to study startup, conduct to close-out.

Find a Partner
Improve your performance with Medidata’s diverse ecosystem.
Ready to streamline your clinical trials process?
Get in touch with our experts today and let’s discuss the ways that Medidata can help support you through all phases of the clinical trial process.