Medidata Named a Leader in IDC MarketScape for Life Science R&D Decentralized Clinical Trial Technology Solutions and Consulting Services
Decentralized Clinical Trials (DCTs)
Inspired by Patients. Powered by Data. Securely Delivered to Scale.
Gain access to the most comprehensive decentralized clinical trial solution built from an ecosystem of tools, people, and processes that let patients, sites, and sponsors participate, contribute to, and monitor any clinical trials outside of a traditional investigative setting.
An End-to-End DCT Solution
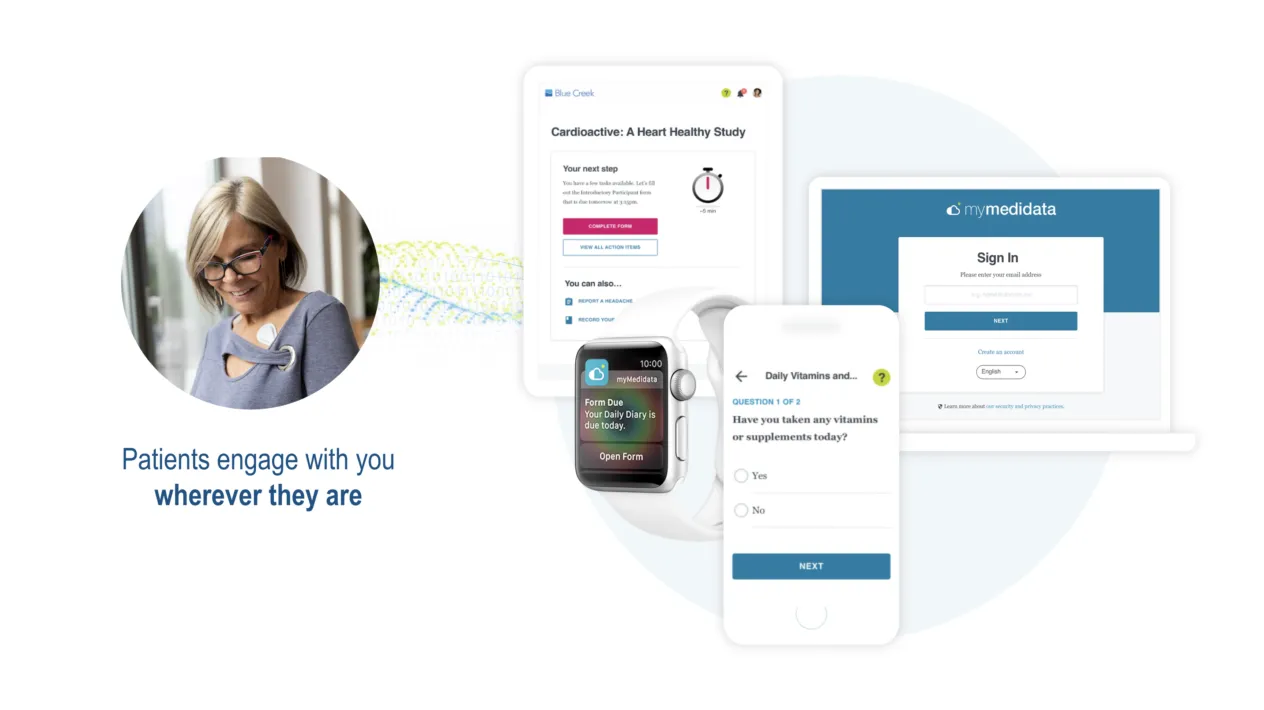
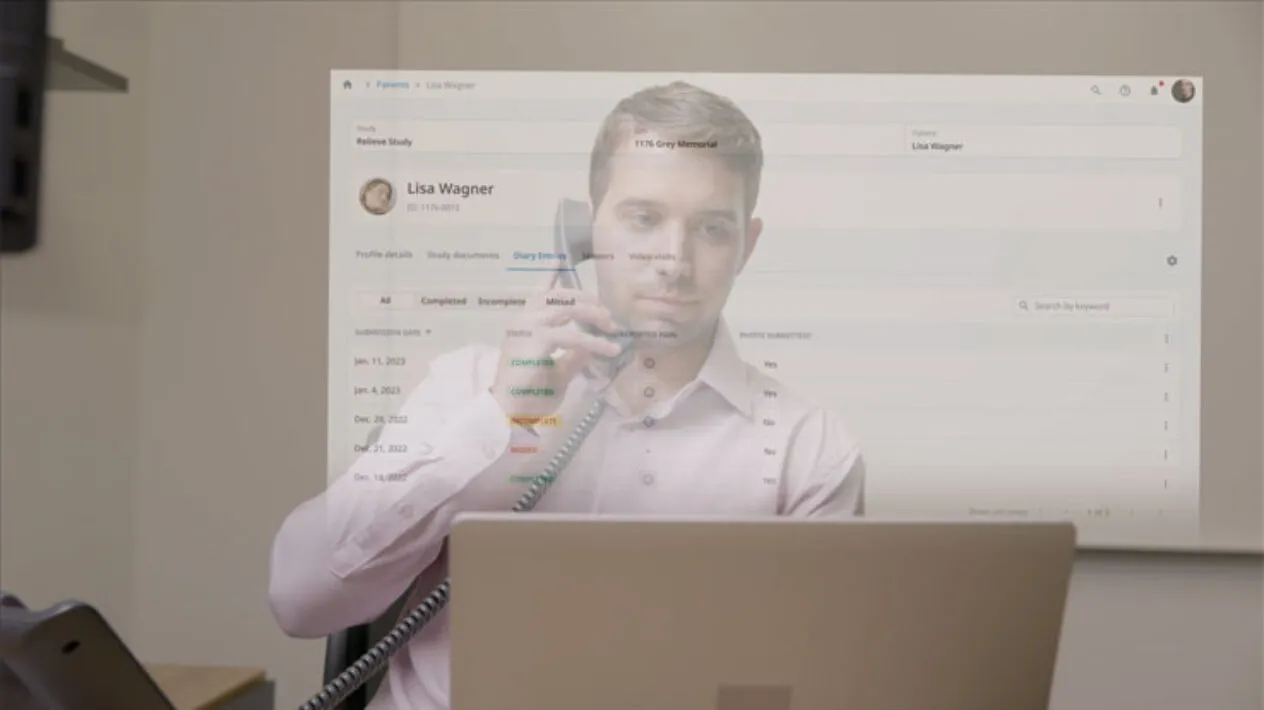
Patient Engagement in Clinical Trials
Patient Cloud is a suite of dynamic, patient-centric solutions that empowers people with choice so you can virtually recruit inclusive and diverse patients, keep them engaged throughout your trial, and produce better study results, and ultimately enhance the patient’s experience.

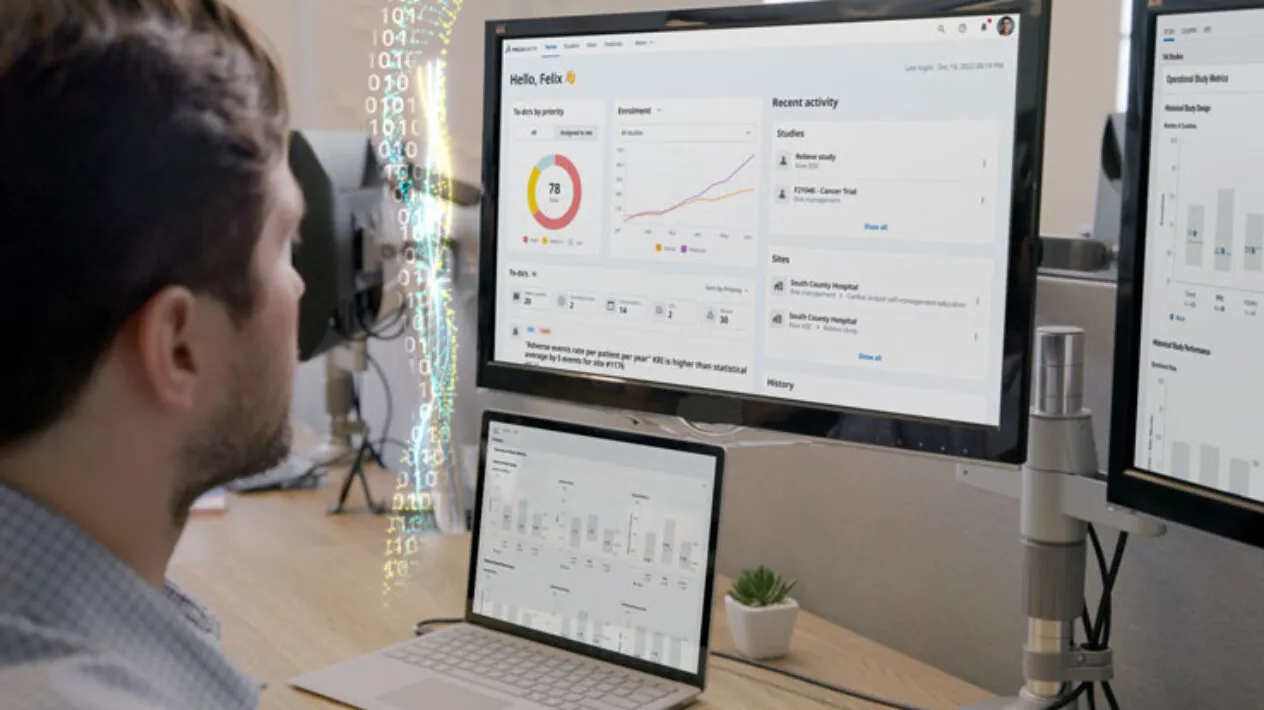
Remote Monitoring and Data Quality Oversight
DCTs present opportunities and challenges for clinical data collection and analysis. Medidata solves these challenges with capabilities for centralized, remote, and risk-based monitoring all on top of a unified data platform. Now you can take a flexible approach to clinical trial monitoring and data quality oversight, shifting from 100% on-site to remote activities, without sacrificing timelines, cost, or data quality.
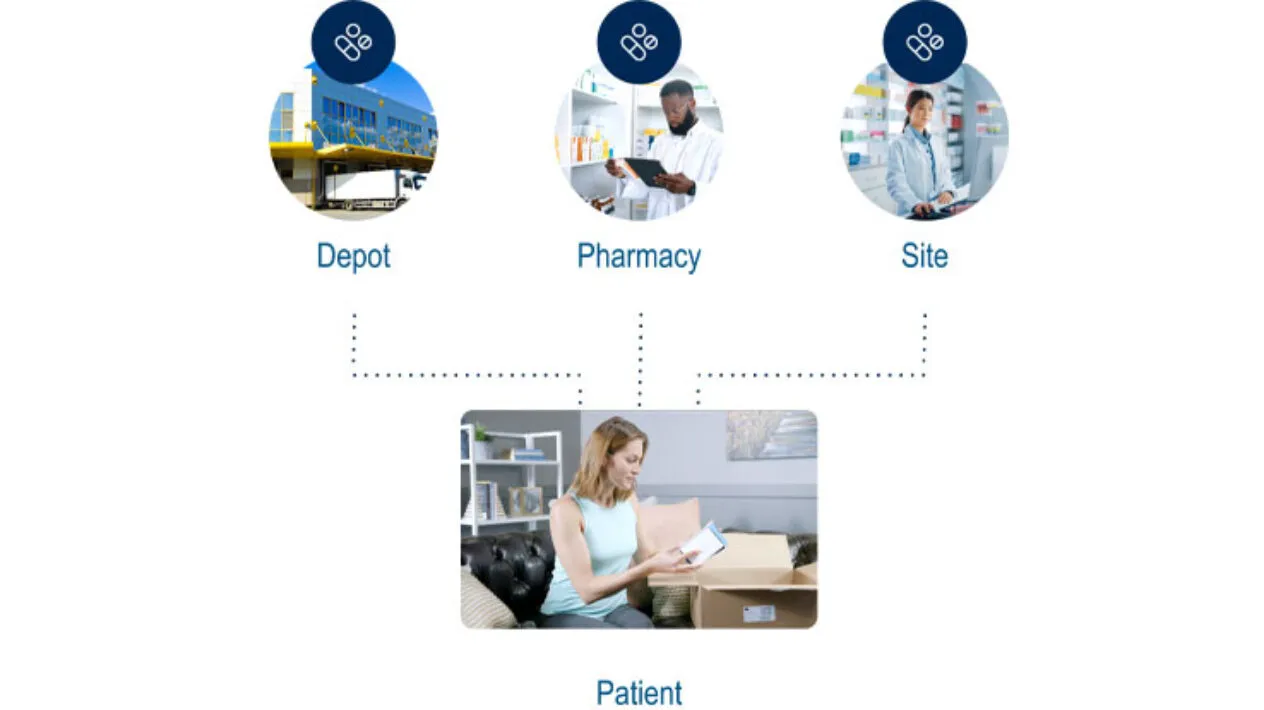
Ease and Flexibility to Execute Direct-to-Patient (DtP) in Real-time
Rave RTSM has unsurpassed DtP capabilities giving sponsors and CROs the highest level of flexibility for decentralized and hybrid clinical trials. DtP can be enabled for any combination of sites and visits and provides sites the ability to determine the source of the patient’s dispensation.
In addition, patients confirm incoming shipments of IP using their myMedidata account.
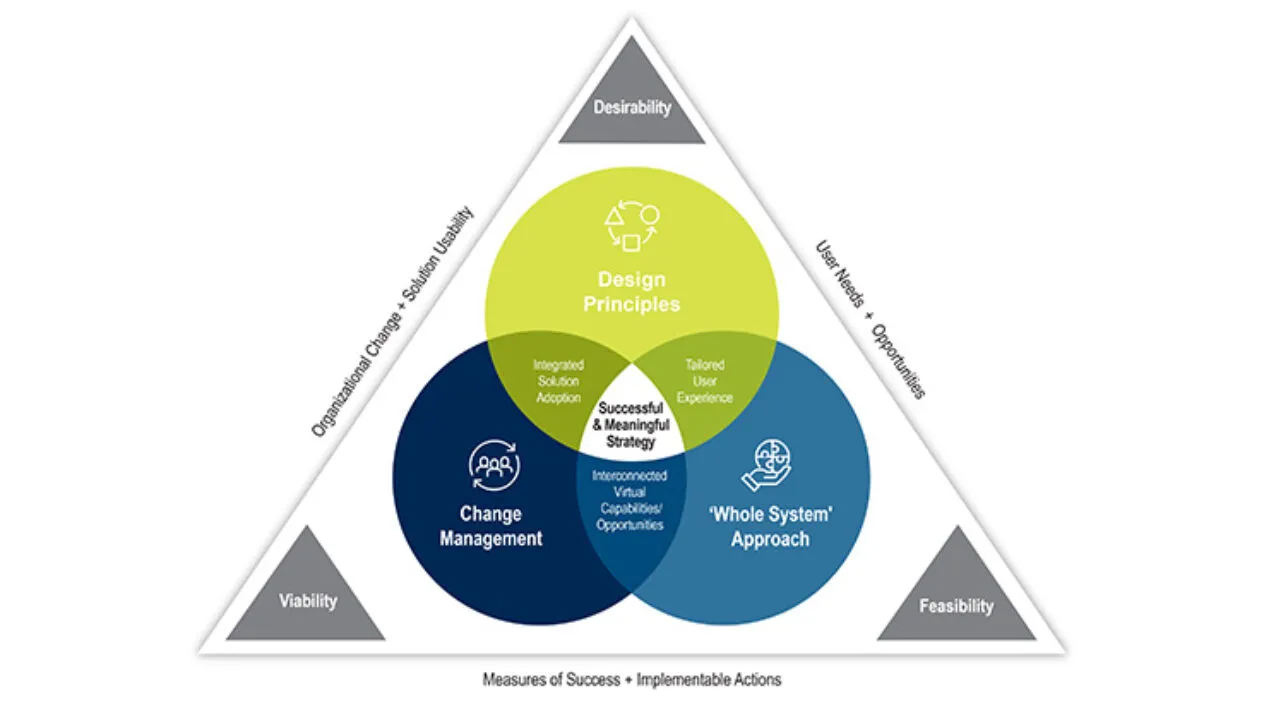
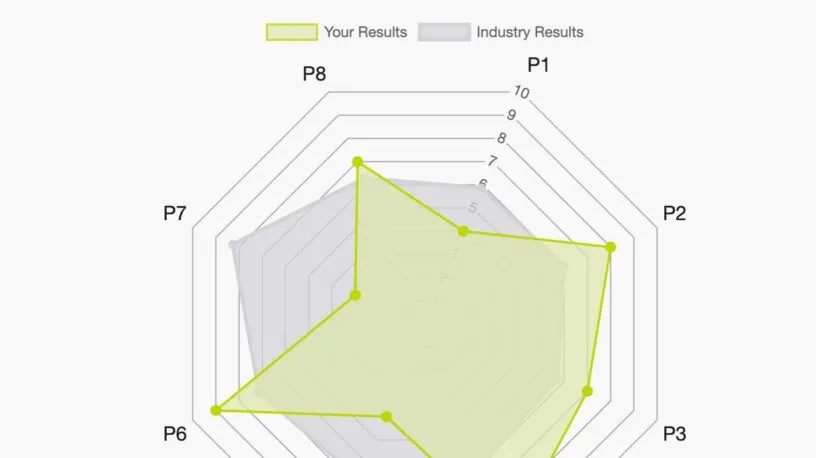
DCT Strategic Design Methodology
Decentralized clinical trials are transforming clinical research by delivering better patient experiences and accelerating drug development. But where do you start?
Our Advisory Services team acts as your personal industry expert in designing and operationalizing your DCT strategies.We take an end-to-end approach by combining the organizational and operational aspects of DCTs to ensure a comprehensive end to end solution. Our goal is to design for both current and future states with the sponsor, patient, and site at the forefront.
What You Need to Know About DCTs
The Medidata Decentralized Clinical Trials Program
The Medidata DCT Program is a scalable, flexible, and comprehensive technology solution to virtualize as much or as little of a clinical trial as needed, including patient participation, data capture and management, monitoring and analysis, and supply dispensation.
Clinical Operations and Decentralized Clinical Trials
Medidata explains the industry’s move towards risk-based quality management (RBQM) approaches to ensure data oversight for DCTs. Discover the value such approaches bring to sponsors, CROs, sites, and ultimately patients.