Medidata Study Builds Accelerate Study Start-Ups
You are looking for ways to accelerate start-up of your clinical trials so you can bring drugs and devices to market sooner. That’s where Medidata Study Build experts come in.
The Medidata Study Build team consists of over 700 global Professional Services experts with clinical industry experience. They gather your requirements and ensure that Medidata’s innovative technology solutions and best practices are properly utilized for long-term success and greater efficiencies.
Accelerate Clinical Trial Study Start-Ups
Accelerate Clinical Trial Study Startups
Selecting the right technology and study build team accelerates your time-to-market, provides greater control of your study data and study execution, and optimizes your entire trial lifecycle. That’s why many life science organizations rely on the Medidata Professional Services Study Build team to achieve the right balance of accelerated clinical trial study start-up, improved data quality, and faster database lock. This allows your clinical trial study team to focus on the patients while we deliver a comprehensive study design that best meets your clinical trial needs.
Leverage Seasoned Clinical Trial Study Build Experts
Medidata’s Study Build experts understand what you are looking for in your protocol design. They have on average over 15+ years of industry experience and understand the need to blend clinical trial best practices with Medidata’s innovative technology to deploy the best possible design. We also understand the importance of working with your internal and external stakeholders to ensure proper integrations and data collaboration for a best-in-class study build.

Don’t Miss a Step on your Rescue Studies
There are times during a clinical trial when change is necessary. Medidata’s Study Build experts understand the challenges involved in a clinical trial rescue study and the importance of solid coordination between the Sponsor, CRO, and sites to minimize disruption. Our experts have migrated studies in all therapeutic areas, from small to large studies in every phase, and from any system. You can trust Medidata’s expertise and experience to make the changes safely and seamlessly.

Maintain your System and Data Ownership
Sponsors want ownership and control over their clinical data. That’s why Medidata provides you with a single source of data ownership for more comprehensive reporting and insights. Medidata experts work with your team to define and create standards resulting in reduced study build times, costs, and resource requirements. Access can also be granted to your CRO partners to reinforce standards and create efficiencies for faster study start-ups.
Benefits of Medidata Study Build Services

Best-in-Class Experience in Technology and Clinical Trials
Medidata’s Study Build team is made up of experts who have come from the clinical trial industry and understand the challenges of building clinical trial studies within a tight timeframe. Your Medidata study build team is led by a project manager, who is your single point of contact to simplify communication and create efficiencies.
Your Study Build experts are trained on the latest Medidata solutions and integration strategies that will accommodate any of your clinical trials – whether a traditional onsite clinical trial or a decentralized one.

Efficient Designs Lead to On‑time Clinical Trial Studies
Medidata’s Study Build experts accelerate timelines by working with your clinical trial team to implement best practices and insights to streamline the study build process. And since your project resources are consistent from one study build to the next, our experts are familiar with your people, processes, and technologies, resulting in faster study builds and reduced delays associated with onboarding new resources.

Drive Downstream Reusable Content
Medidata’s Study Build team also provides users with the ability to streamline future clinical trial study builds.
Medidata has created an easy-to-use Global Library where you can access forms as needed. It also enables higher re-use of electronic Case Report Forms (eCRFs), decreasing study build times and driving standards across the entire workstream. This allows clinical study builds to be faster and more cost-effective – in line with your standards.
Tested and Proven Success Measurements
Our Study Build teams have decades of experience in building successful clinical trial study builds to meet your needs and requirements. We have found that by leveraging our Study Build services you can achieve study milestones in record time.
Reduce Your Study Builds by
ONE MONTH
When using Medidata Professional Services
Conduct Studies
FIVE MONTHS
Faster when using Multiple Products
Reach Database Lock
FOUR DAYS
Sooner when using Multiple Products
- Analysis of difference in median build time vs. matched studies not using PS (p<0.05); Reduction of 30 days.
- Analysis of difference in median FPI to LPLV time for EDC + at least one additional product vs. EDC only studies (p<0.05) 2017 to 2021; Reduction of 59 days.
- Analysis of difference in median LPLV to DBL time for EDC + at least one additional product vs. EDC only studies from 2017 to 2021.
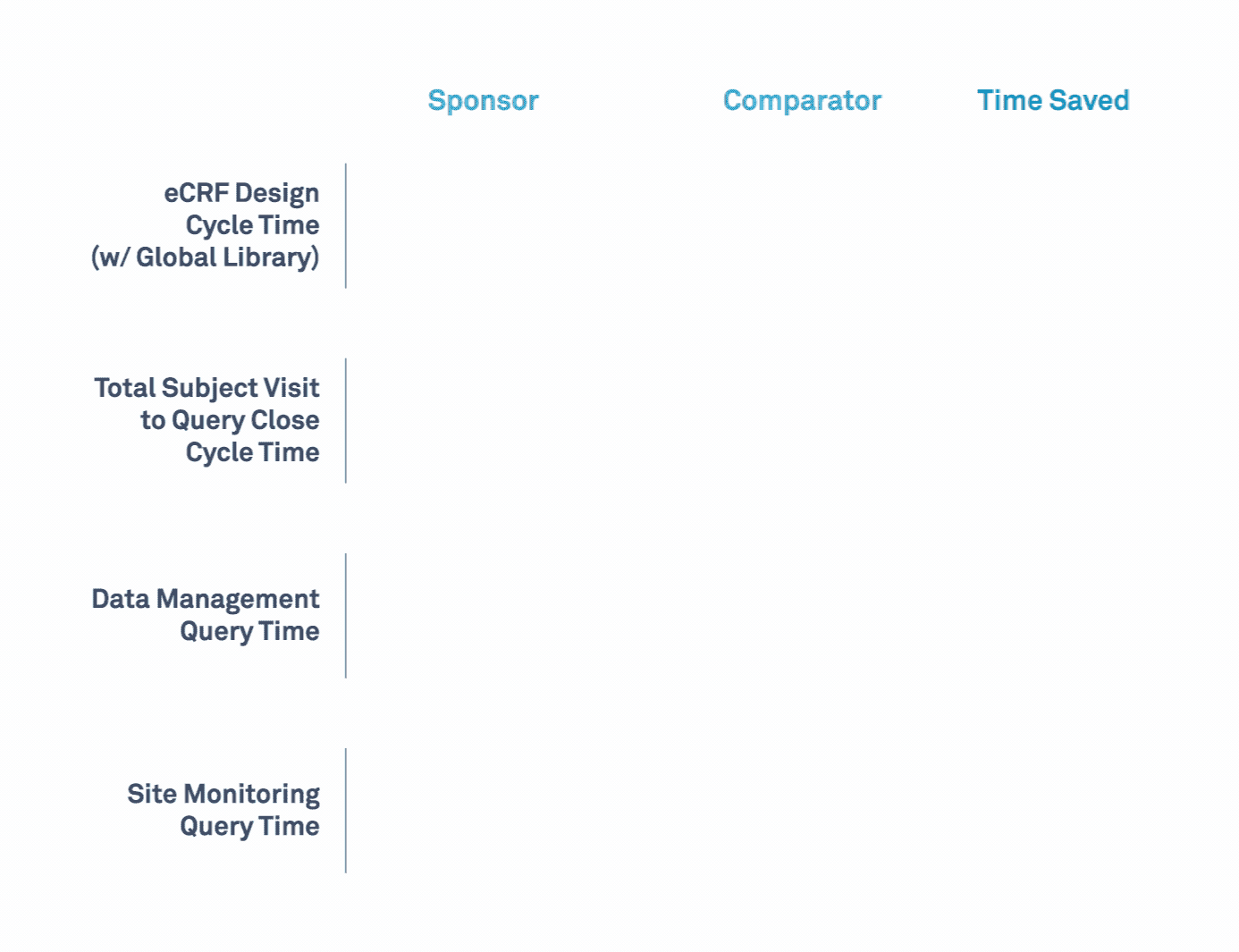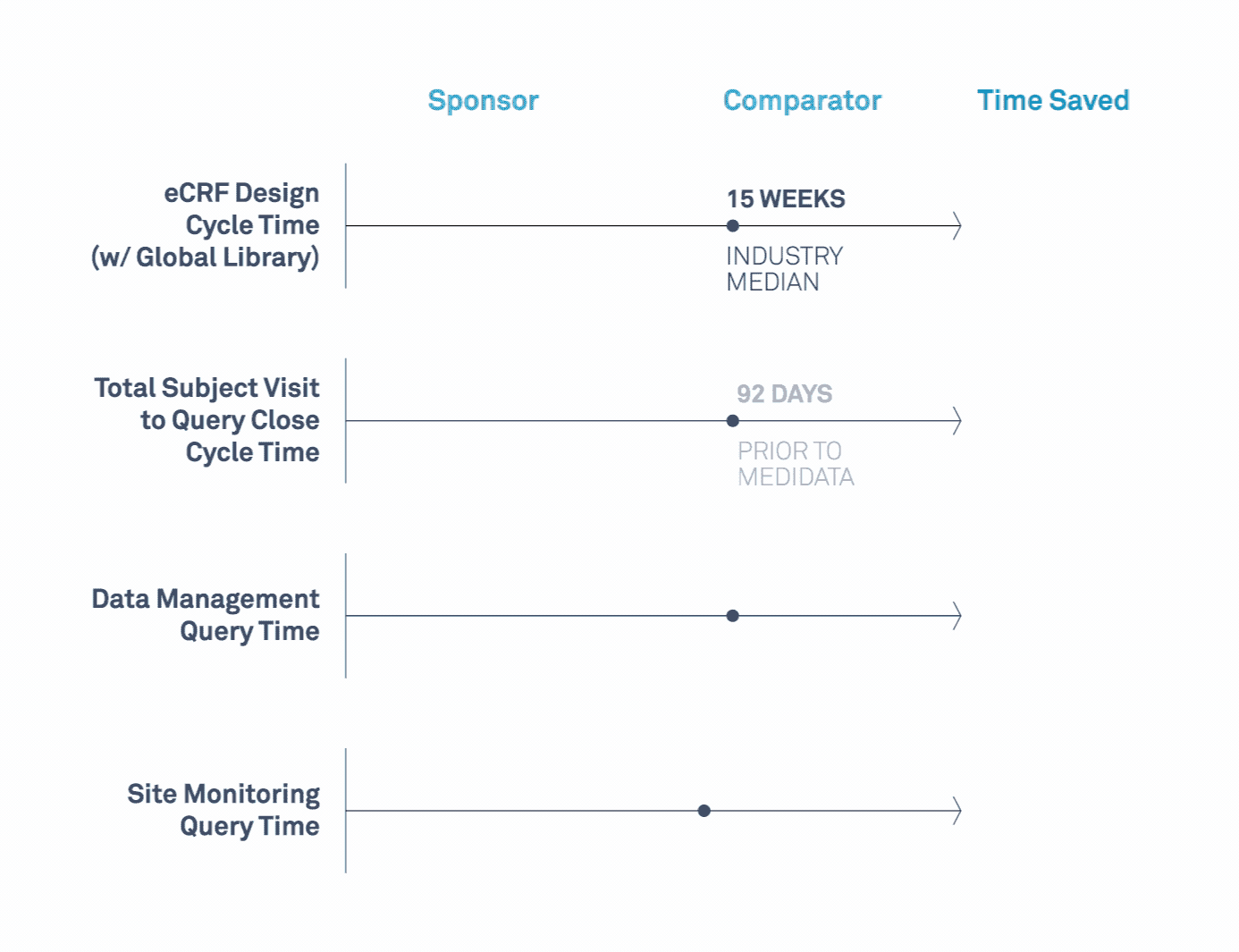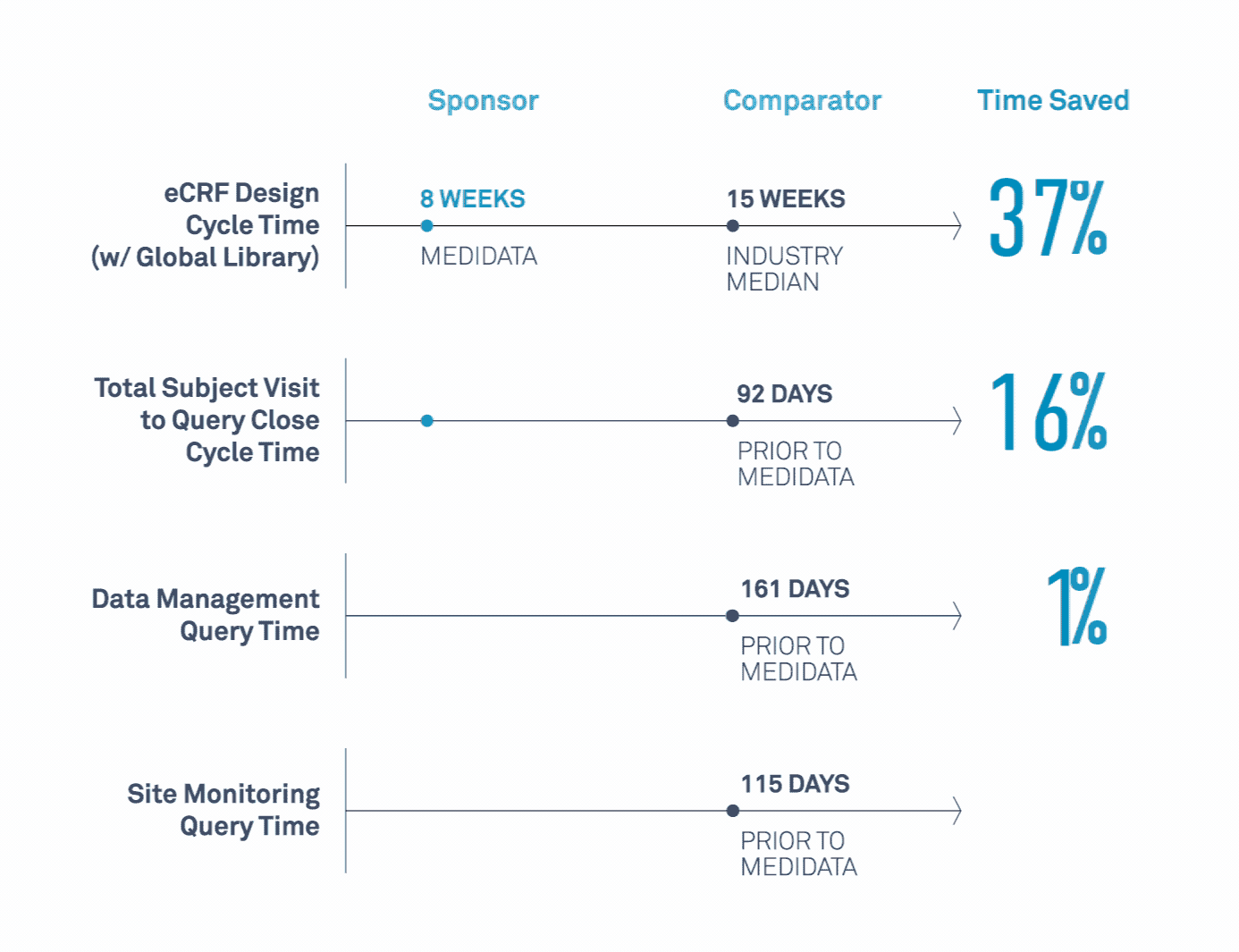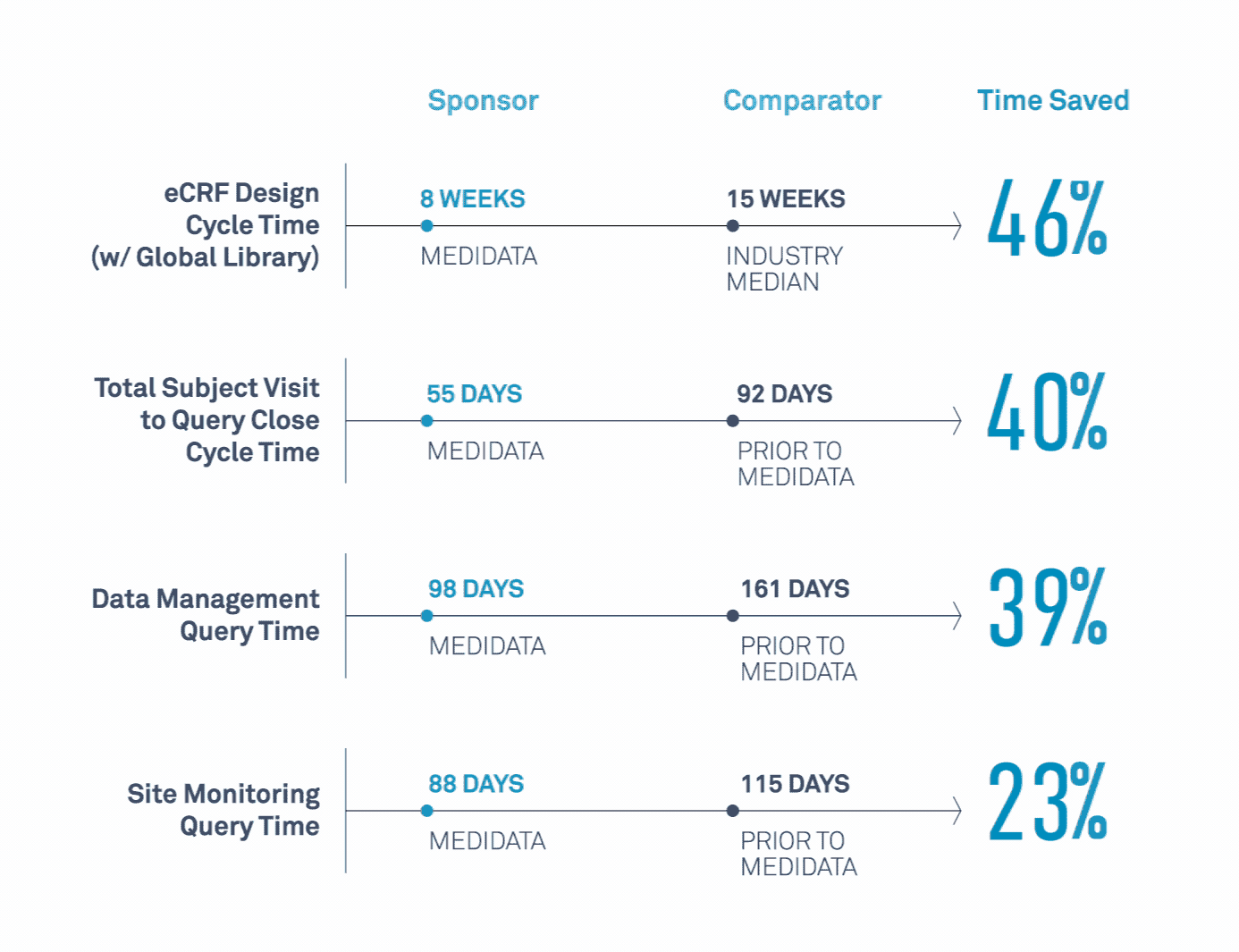
Medidata’s Study Build Team Helps Transform Clinical Trials
Learn how a leading biopharmaceutical company maximized value related to its study design, data cleaning, data quality, and site monitoring processes.
DISCOVER NOW
Let’s talk. Learn how Medidata Study Build experts can help you accelerate your study startups.