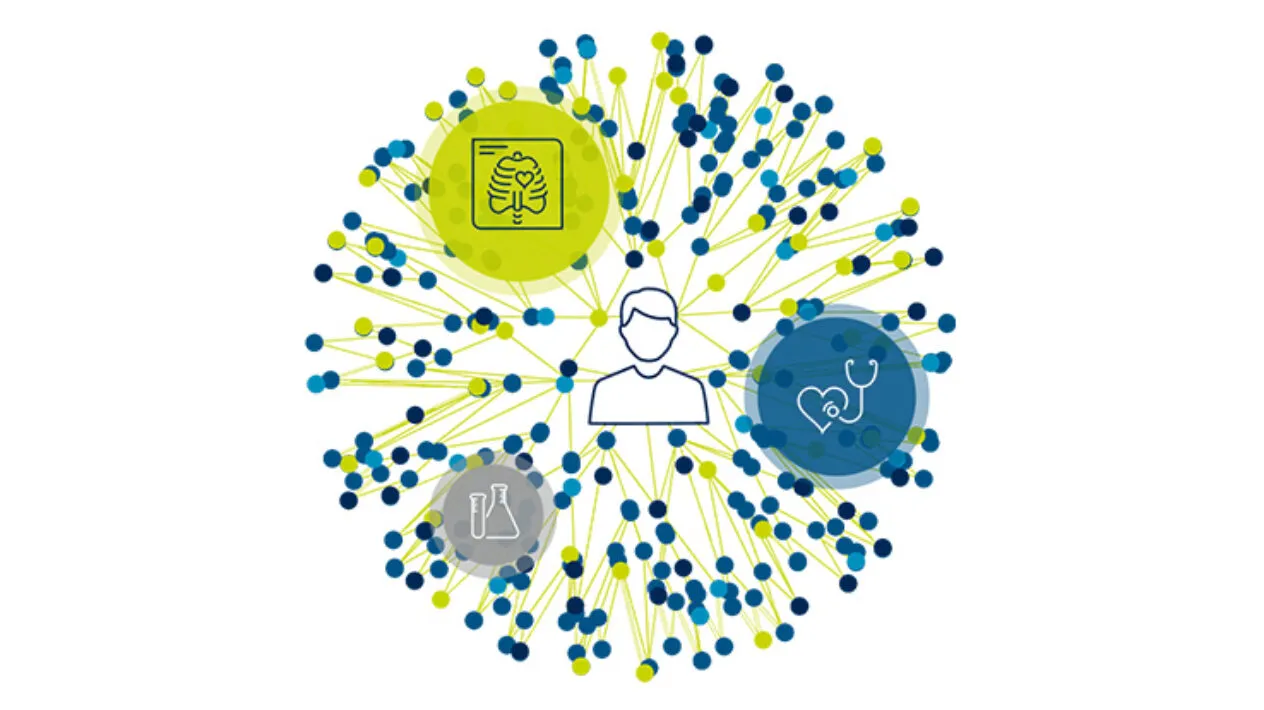
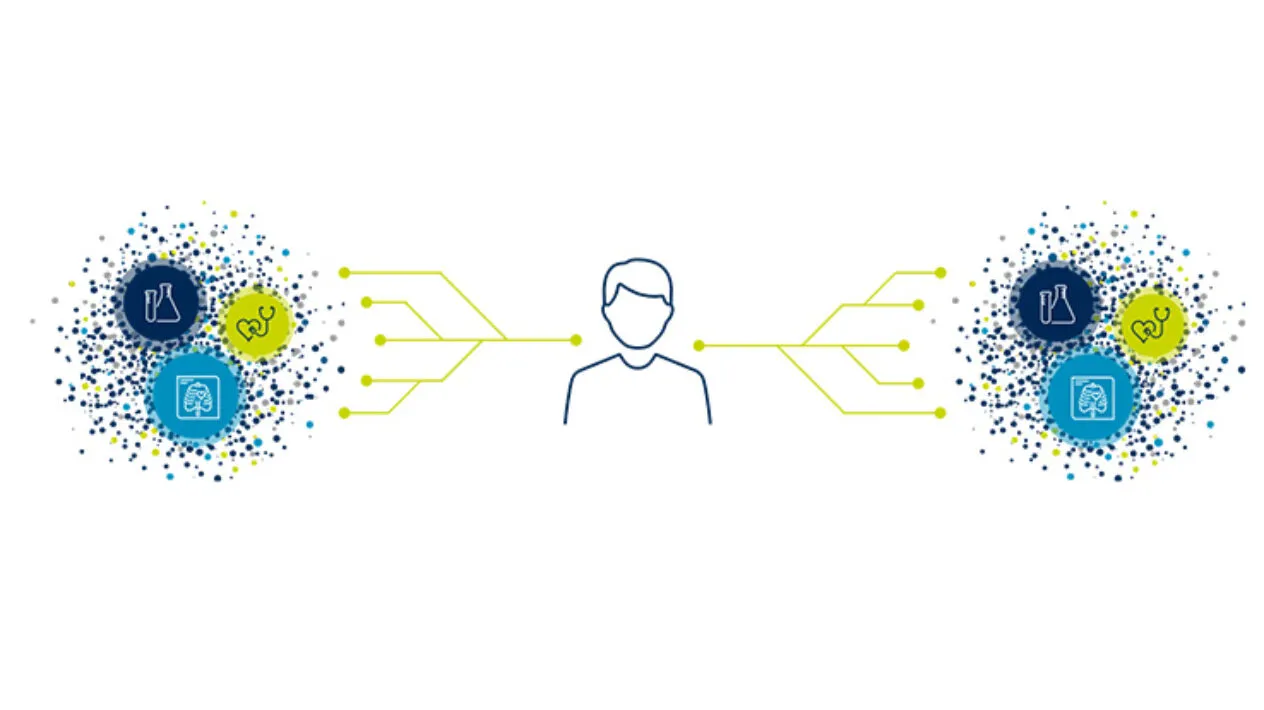
Clinical Trial to Real-world Data Linkage
Medidata Link enables clinical trial data linkage with real-world data (RWD) at the patient level to enhance evidence generation beyond what is possible in traditional clinical trials. By creating an enriched view of the patient journey before, during, and after your trial’s completion, Medidata Link generates insights that enable you to accelerate patients’ access to innovative treatments while minimizing site burden and costs.
Better Together: Clinical Trial Data to Real-World Data Linkage
Enable Long-term Follow-up
Leverage trial patients’ RWD to collect data on long-term effectiveness and safety outcomes, even after trial completion, without adding burden to patients or sites. This can facilitate post-market studies, long-term effectiveness studies, and long-term safety monitoring studies.
Mitigate Loss To Follow-up
Mitigate the impact of loss to follow-up on your trial end points by monitoring patients activity through their routine care data and gaining expanded insight into their treatment and outcomes when they don’t return for scheduled follow-up.

Enable Label Expansion Studies
Provide evidence to support label expansion using additional patient variables and outcomes from linked RWD sources.

Improve Confidence in External Control Arms
Analyze RWD of intervention arms to more accurately compare outcomes between external control arms built on RWD and intervention arms.

Demonstrate Economic Value
Demonstrate economic value in payor and provider discussions by measuring the cost of care and healthcare resource utilization for trial patients before, during, and after your trial.
Key Features

Easy Implementation
Medidata Link seamlessly integrates into your trial workflow to collect and ingest personally identifiable information via paper, eConsent, or Registry routes with frictionless patient or site-facing data collection tools. It is easy to implement and can reduce data collection burden on patients and study sites.

On-Demand Trial Linkage to RWD
Medidata Link gives you full flexibility in your choice of linkage approach, supporting a wide range of RWD sources and tokenization vendors. As Medidata centrally manages personally identifiable information it can also support trial linkage to RWD sources through de-identified (tokens) or identified (patient identifier) based workflows.

Compliant, Up-to-date Data
Medidata Link lets you robustly manage clinical trial data linkage consent status to meet IRB and GCP requirements, and supports consent withdrawal even after the trial concludes and sites become inaccessible.

Site Enablement
Medidata Link provides comprehensive training materials to sites and enables seamless implementation within sites’ existing workflows for an out-of-the-box launch.
Related Products
Learn More

10 Data Linkage Use Cases to Future-Proof Your Clinical Trial
In this eBook, we discuss Medidata Link use cases in varied indications across the clinical development lifecycle, including:
- Tracking Patients Lost to Follow-up
- Reducing Patient Burden and Augmenting Decentralized Clinical Trials
- Monitoring and Contextualizing Patient Reported Outcomes
- Quantifying Healthcare Resource Utilization
- Evidence for Label Expansions
- Long-term Safety and Effectiveness Tracking
- Use in Expedited Programs
- Support Regulatory Grade Pragmatic Trials
- Improve Quality of External Control Arms (ECAs) Built Using RWD
- Enable Head to Head Comparison Not Studied in Clinical Trials

Featured Webinars
Watch our latest webinars on trial linkage, clinical trial tokenization, and RWD to learn how connecting clinical trial data with RWD can help create a holistic view of the patient journey.

Featured Blogs
Read our latest blogs on trial linkage, clinical trial tokenization, and RWD to learn how connecting clinical trial data with RWD can help create a holistic view of the patient journey.