The Changing Landscape of Clinical Trial Monitoring
Clinical Operations is evolving at the pace of the research it supports. Optimized clinical trial operations are a key differentiator for delivering high-quality treatments, on-time and efficiently, but the landscape is changing. Large scale decentralized clinical trials (DCT) which acquire high-volume data from multiple sources have permanently changed the paradigm for clinical monitoring towards remote and risk-based approaches.
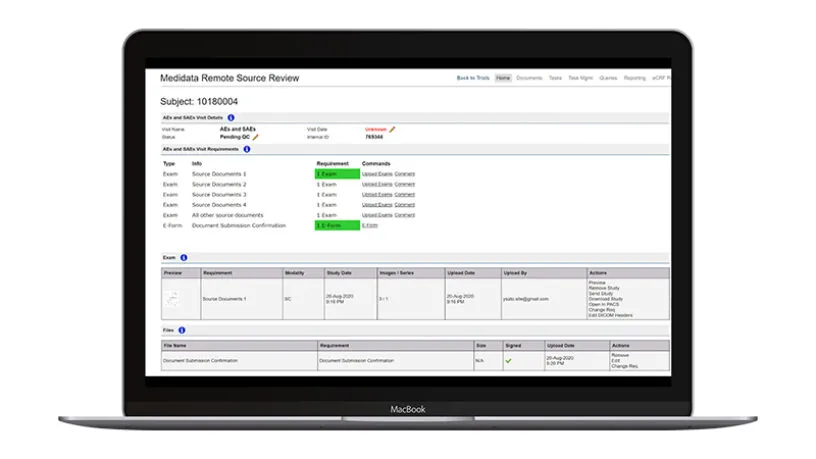
More and more research organizations are turning to modern monitoring techniques such as risk assessments, central monitoring, reduced SDV/SDR, and remote source document review.
Learn More

The Changing Landscape of Clinical Monitoring
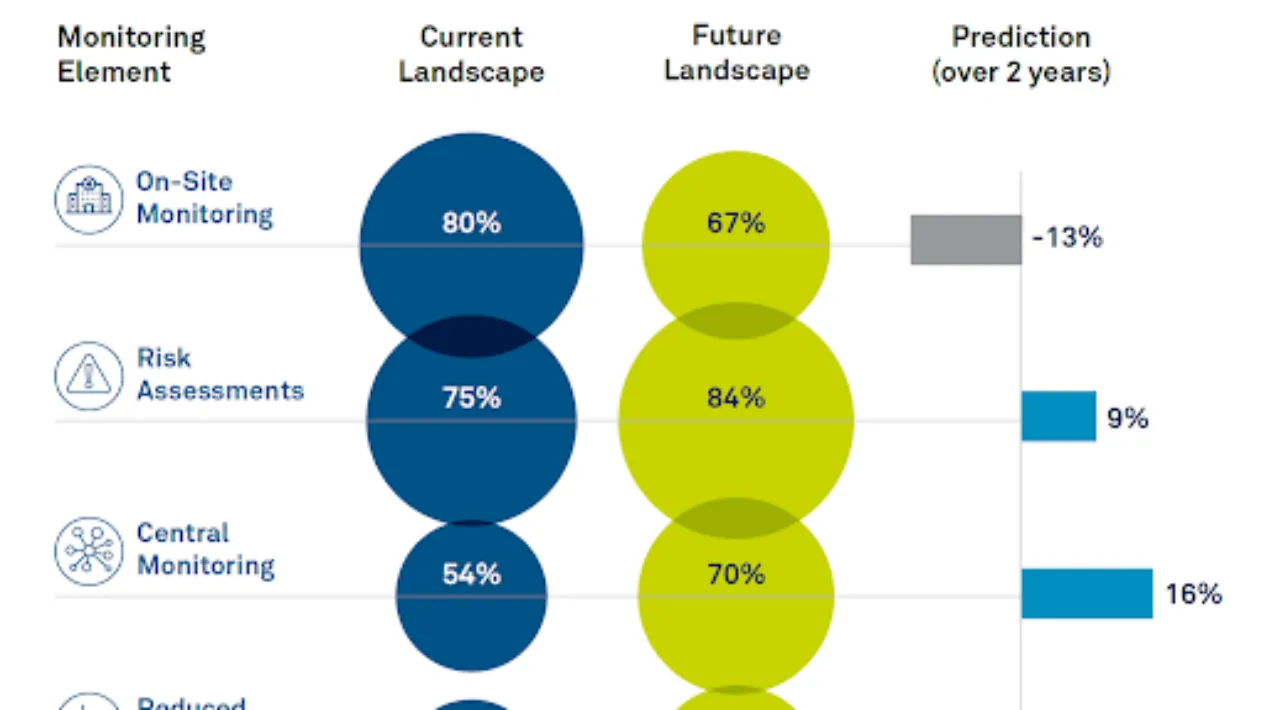
Medidata recently commissioned an independent industry survey to understand the current landscape for clinical monitoring modalities and expectations for the future.

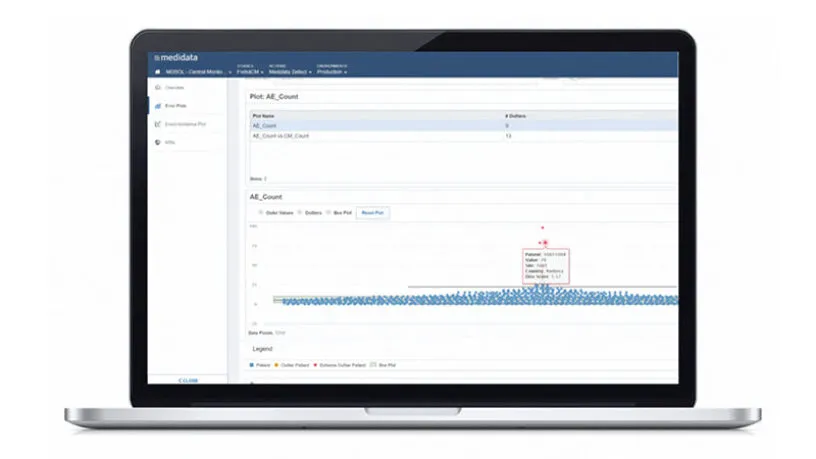
Improving Clinical Trial Oversight with Central Monitoring
Read this white paper to learn how proper adoption of risk-based quality management strategies, including centralized monitoring, can shorten trial timelines, reduce overall costs, and improve trial outcomes.

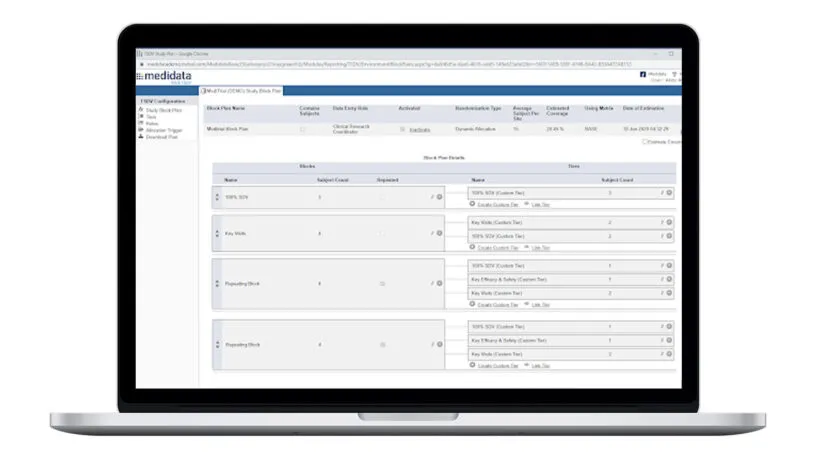
Plan and Execute Better SDV Strategies
Rave TSDV allows you to easily design, configure, and execute a highly targeted SDV strategy and still maintain full coverage of critical safety and efficacy data.
Plan study-specific and site-specific SDV all the way down to the data field, form, and patient visit.