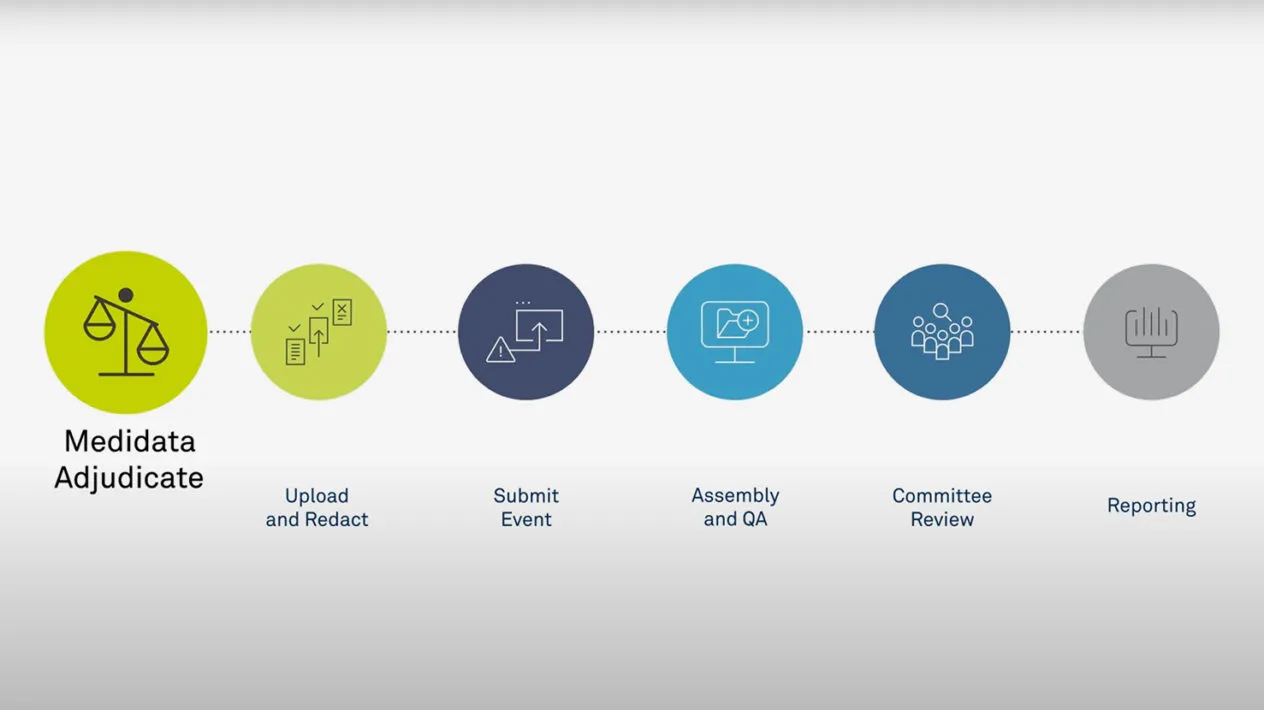
Medidata Adjudicate
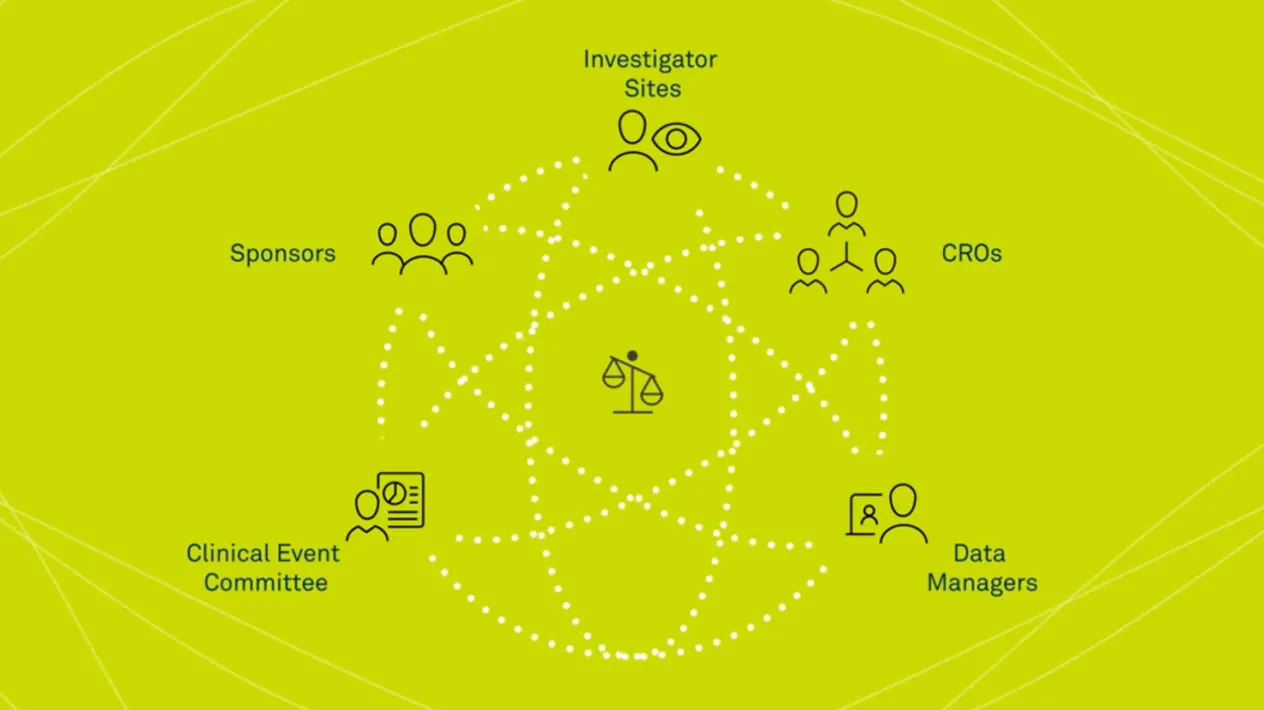
An effective Clinical Event Committee (CEC) reduces variability in adjudication outcomes, ensuring that the best outcomes are collected in your trial. Having the right endpoint adjudication technology allows you to trace every aspect of the adjudication event, from collection, de-identification and dossier aggregation to committee review and management, giving you control and visibility of all your events.
Medidata Adjudicate is a state-of-the-art clinical endpoint adjudication system, fully integrated with the Medidata Platform, providing the unique ability to function as a one-stop shop for all your clinical trial needs. A single solution that follows all clinical events from beginning to final outcome, Medidata Adjudicate is designed to support investigator sites, sponsors, CROs, data managers, and the CEC who collect, manage, organize, adjudicate and submit clinical endpoint data.
Optimize Endpoint Adjudication

Efficient Clinical Trial Adjudication
Medidata Adjudicate provides quick, easy study implementation with self-service configuration. Built-in web-based pdf editor capability supports the creation of dossiers, including PHI masking, labeling, bookmarks, and organization of dosser elements for efficient management of your complex adjudication needs.

Unified Platform
Medidata Adjudicate is part of the proven, scalable, and unified Medidata Platform, providing the benefits and ease of one technology for all your trial needs. With real-time visibility into and across all events and status, you will realize a reduction in duplicate clinical data entry and workload.

Flexible Workflows for Any Adjudication Committee Design
Optimize tasks and ensure clinical data quality and timely review with intelligent, flexible workflows. Leverage multi-faceted communication tools for additional data requests and queries on a platform that accept all data types to support any end point design and adjudication committee design (e.g., double review with adjudication, triple review, consensus, etc.)

Easy-to-Use Clinical Trial Technology
Medidata Adjudicate offers simple, intuitive navigation of dossier review and task lists for efficient CEC review. The ease of a single sign-on through Medidata’s leading cloud-based technology reduces site burdens of time and effort.

Clinical Study Support
Implement with confidence. Medidata’s Professional Services team of experts has countless years of clinical trial experience and a deep understanding of the industry. We partner with you every step of the way to ensure your adjudication processes are optimized so you can achieve the highest value.
Key Features of Medidata Adjudicate
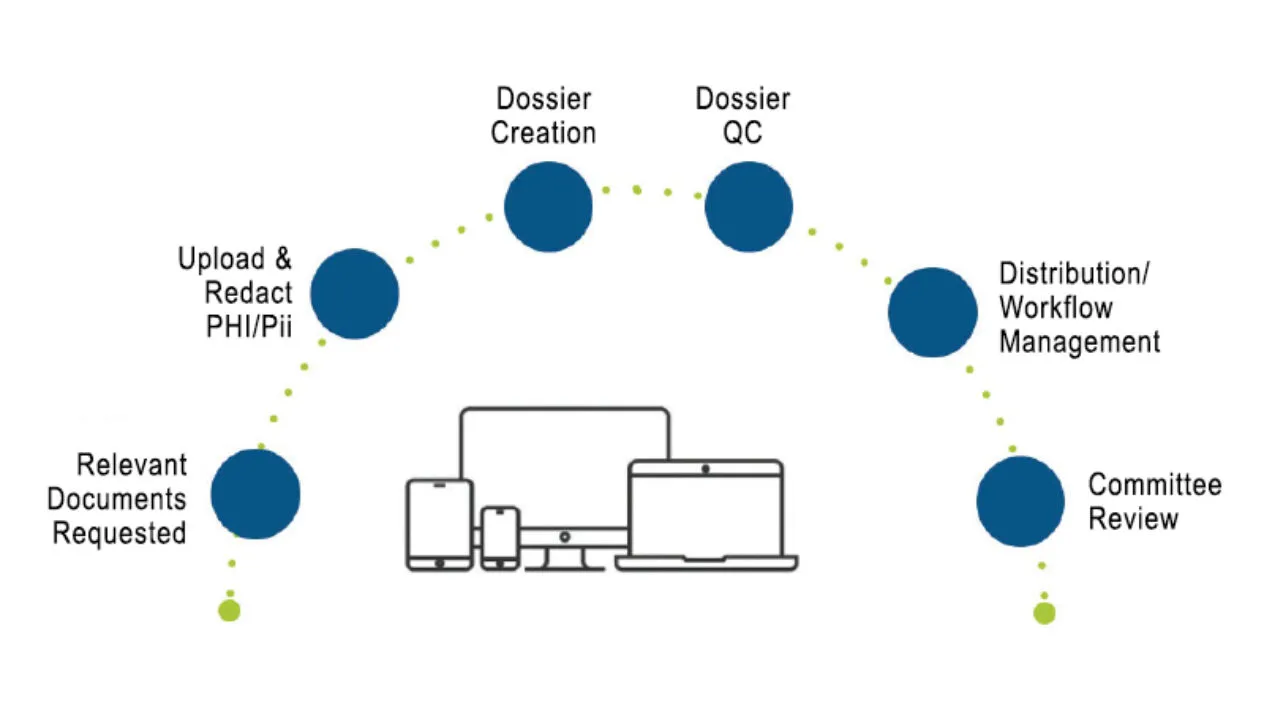
End-to-End Clinical Adjudication
Medidata Adjudicate offers comprehensive capabilities to support clinical trial adjudication event management, including customized workflows per event type, event status visibility and reporting, self-service configuration, web-based dossier creation and editing tools, and flexible communication options (e.g., additional data requests and queries with configurable query templates).

Clinical Endpoint Committee (CEC) Adjudication Support
To optimize CEC review, Medidata Adjudicate provides easy navigation of dossier for efficiency, optimized workflow and assignments for timely delivery of work, automated triggers for committee member discordance, side-by-side form comparison for easy consensus review, and autosave capability to ensure no work is lost.

Site Features
Ease your clinical trial site’s burden with Medidata Adjudicate. Functionality including easy upload of any clinical trial data type (including documents and medical images), automatic subject creation with EDC unification, built-in PHI redaction with a search and redact feature, automated query reminders with ability to view and respond, and a cloud-based integration that eliminates software installation, reducing clinical trial site time and effort.
Related Solutions
Learn More
Is it Time to Transform Your Endpoint Adjudication Process?
Having the right endpoint adjudication technology allows you to trace every aspect of the event, from collection, document de-identification and dossier aggregation to committee review and management, giving you control and visibility of all your adjudication events.

Transform Your Drug and Device Safety Data & Adjudication Processes with Clinical Trial Technology
Integrated, flexible cloud platforms can streamline your clinical endpoint adjudication process, cutting costs, improving data quality, smoothing regulatory approval, and speeding time to market.

Introducing Medidata Adjudicate
Transforming your traditional error-prone manual processes or disparate technology set ups to a streamlined, single digital endpoint adjudication system, brings efficiency, transparency, and accuracy to the process.
How Technology is Transforming Clinical Endpoint Adjudication
As with many other areas of clinical research, innovative new technologies – when combined with scientific and medical expertise – are helping to remove barriers and drive significant gains in efficiency, accuracy, data quality, and compliance with clinical endpoint adjudication. Learn more from the experts in this on-demand webinar.