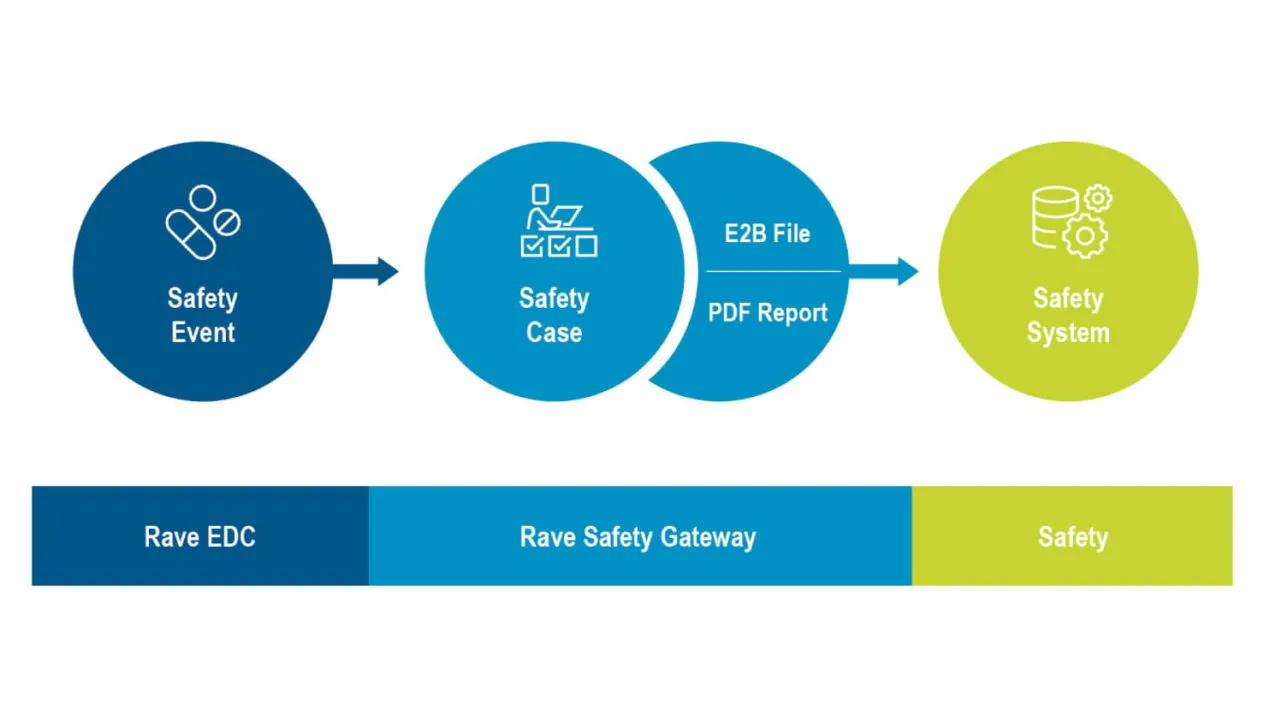
Rave Safety Gateway
Medidata Rave Safety Gateway, part of the Medidata Data Experience, is a secure, configurable interface that transmits AE data from Rave EDC to external E2B-compatible safety systems. This provides an automated clinical data capture, tracking, and transmission process, resulting in fewer errors, less data reconciliation, and improved patient safety.
Why use Rave Safety Gateway?
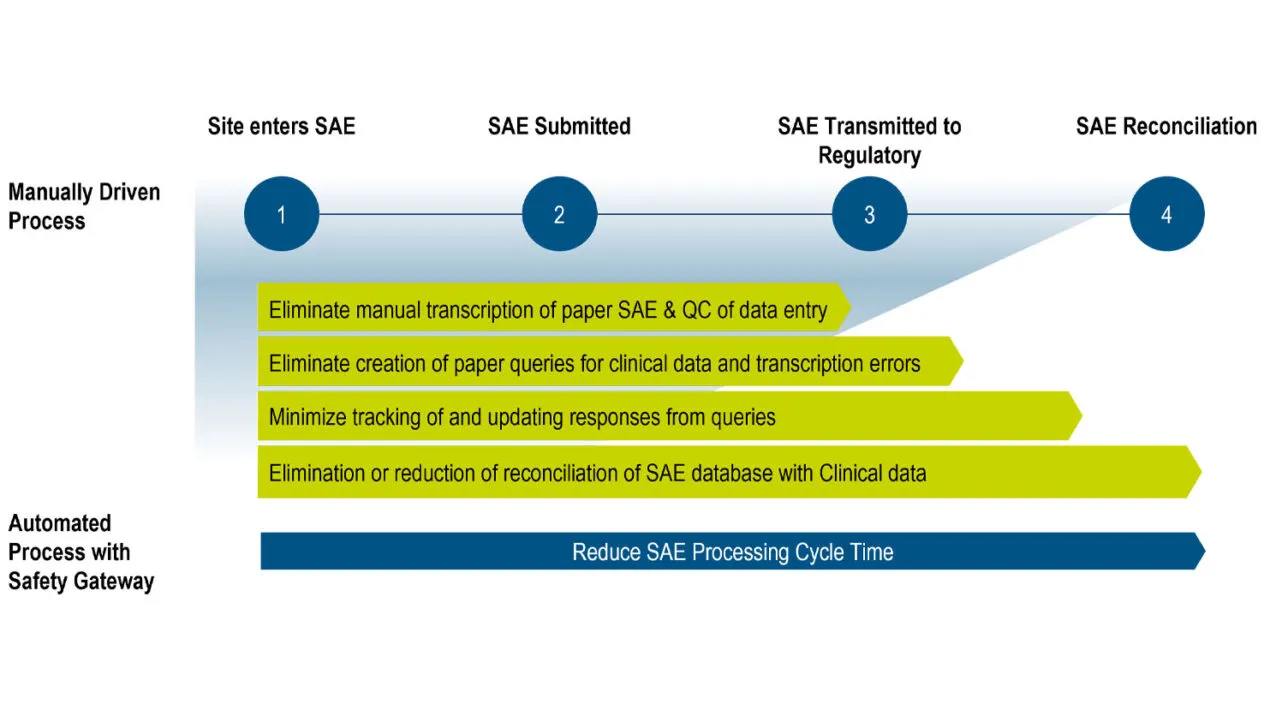
Reduce Burden on Sites and Data/Safety Teams
Automatically deliver data from Rave EDC forms into your safety system with Rave Safety Gateway.
Reduce the burden of adverse event reporting on your clinical trial sites, and the clinical safety data management query and reconciliation efforts of your data managers and safety teams.

Improve Accuracy of Clinical Safety Data Management
Significantly reduce manual AE reconciliation and the risk of errors in safety case data. Transcription errors are eliminated as AE data is entered once into Rave EDC and automatically transmitted to your safety system.
Verbatim terms in AE reports are also coded using Rave Coder with 98% accuracy in automatically suggested coded terms.

Accelerate Transmission of Safety Case Data
Eliminate duplicate entry and manual transmission of safety case data, and reduce AE reconciliation, data review, and query cycle times.
If AE data in Rave EDC is not signed off within a specific period, Rave Safety Gateway lets you choose whether to always automatically transmit the data to the safety system, including updates to existing AE reports.
Key Features of Rave Safety Gateway

Use with Any ICH E2B Compliant Safety System
Use Rave Safety Gateway with any ICH E2B R2 or R3 compliant safety system to improve your clinical safety data management. Safety Gateway collects and prepares your safety data into a file in the industry-standard ICH E2B R2 or R3 format ready for import into your safety system.

Manual, Automatic, and/or Timed Transmission
Manage safety case data transmission your way. Rave Safety Gateway lets you choose whether data is automatically exported into E2B files or sent for review, selection and approval before export. Timed Trigger Transmission is a fail-safe mechanism to ensure timely reporting of AEs. If, after a predefined time, AE data has not been signed off, it can be configured to be automatically transmitted to your safety system.

Configurable Mapping of Safety Case Data
Choose what Rave EDC data is transmitted to your safety system and which safety case elements it is mapped to.
Rave Safety Gateway lets you control the mapping of fields in Rave EDC to ICH E2B R2 and R3 elements, monitors those fields to detect data entry, and prepares the E2B file for transmission to your safety system using those mappings.

Automatic Notifications
Alert your safety team to new safety case data, so that no time is lost in your safety reporting process.
When data is received or exported by Rave Safety Gateway, and the receipt of the ICH E2B file by the safety system is acknowledged or unacknowledged after a defined period, user groups or individuals can receive email notifications to alert them to these events.
Related Solutions
Learn More

Fact Sheet
This white paper provides a summary of why Clinical Data Management (CDM) must quickly adapt to the mounting data pressures in modern clinical trials and discusses the three pillars that form the foundation of a modern intelligent CDM platform that is needed to succeed in an increasingly complex clinical trial world.

Global Pharma Bridges the Gap Between Data Management and Pharmacovigilance with Rave Safety Gateway
As a global organization, the sponsor needed a more efficient way to ensure its safety data was captured cleanly and with reduced redundancy through the use of a single data source with up-to-date SAE information and more formal control over data entry, as well as a more collaborative, better-flowing process between the clinical data management (CDM) and PV teams. Read how they achieved this with Rave Safety Gateway.

Modernizing Clinical Data Capture and Management for Today’s and Tomorrow’s Trials
The clinical data landscape continues to evolve, but the way we manage that data has not kept pace with the growth in data sources, types, volume, and velocity. This infographic explores the challenges and proposes a solution to modernize clinical data capture and management.



