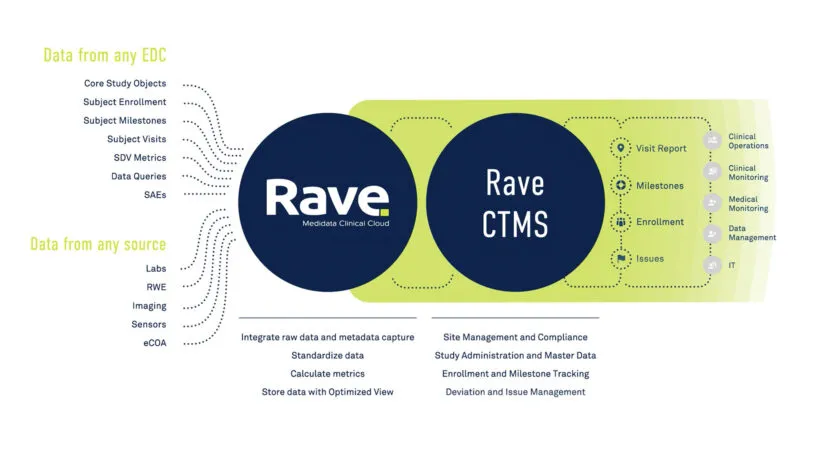
Intelligent Trials
Increase confidence in study planning and execution to deliver treatments to patients faster. Our AI-powered solutions provide unmatched site-level granularity and actionable real-time insights, so you can accelerate trial timelines, prevent enrollment delays, enroll more diverse patients, monitor real-time study performance, and ensure the success of your clinical program.
Benefits of Clinical Trial Analytics
Identify Study Footprint
Determine realistic timelines and study footprint by assessing enrollment rates of industry trials and selecting which countries and sites would be optimal to meet timeline goals..
Select High-Performing Sites
Improve site selection even before you start your trial to meet enrollment timelines by leveraging industry-wide, real-time, and historical enrollment performance data, supported by predictive analytics and enrollment forecasting.
Learn more about Intelligent Trials Study Feasibility.

Enroll More Diverse Patients
Ensure your trials reflect the diversity of real-world populations by selecting sites with a proven track record of recruiting diverse patients. Our indication-specific, cross-industry data allows you to identify and prioritize sites that consistently meet diversity goals, helping you achieve your inclusivity targets and drive equitable clinical outcomes.
Learn more about the Intelligent Trials Study Feasibility Diversity Module.
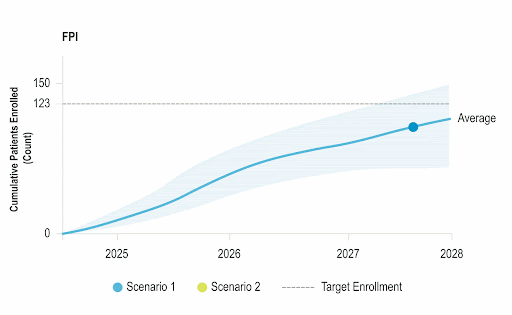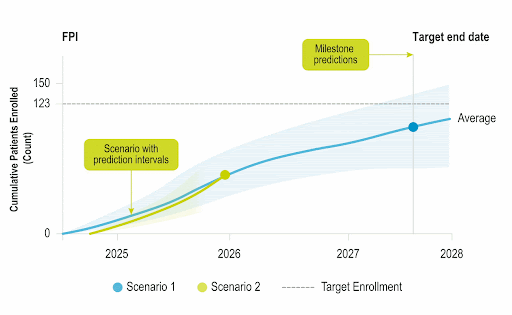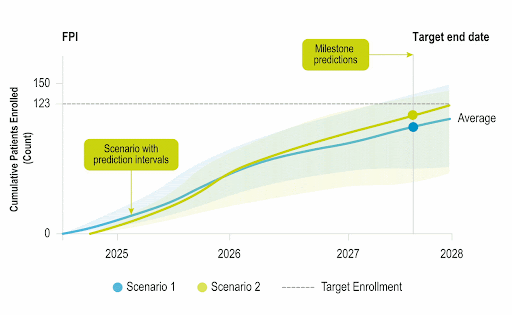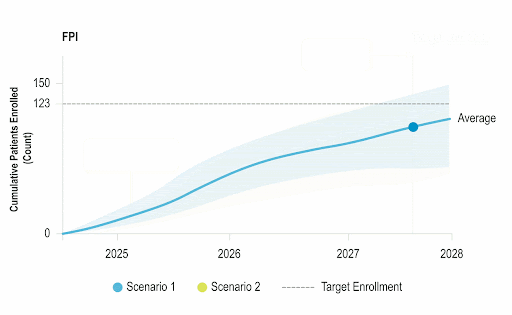
Accelerate Enrollment
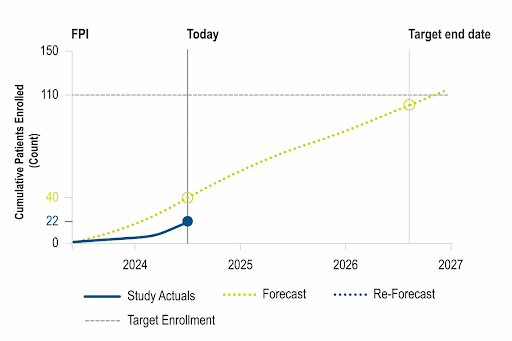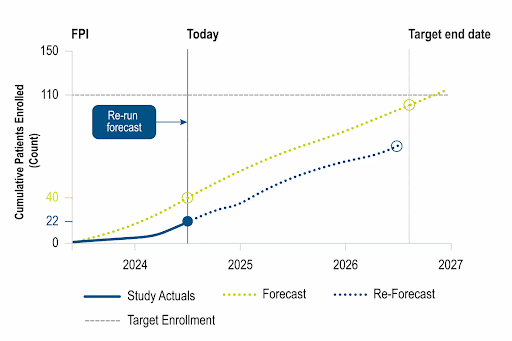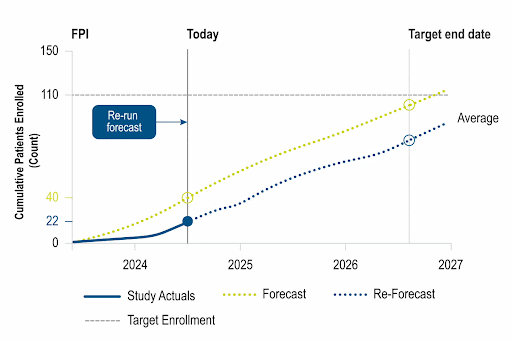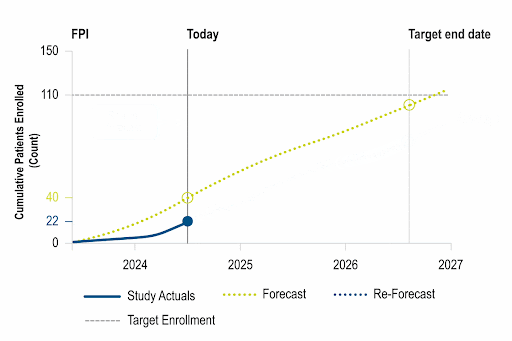
Identify opportunities to improve operational performance and accelerate enrollment by comparing your trial to the performance of industry trials and forecasting enrollment.
Learn more about Intelligent Trials Performance Analytics.
Key Features
Industry’s Largest Performance Dataset
Cross-pharma and CRO dataset that spans over 33k trials and 10M patients, adding unique value to even our largest customers.
Unmatched Site-Level Granularity
Metrics span operational and diversity performance, enabling selection of sites that will accelerate the study and increase clinical trial diversity.
Actionable Real-Time Insights
Unique insights leveraging data refreshed weekly from ~8k active studies, allowing customers to calibrate performance and take action within a competitive landscape.

Turnkey with White-Glove Support
Off the shelf SaaS solution and API access combined with white glove service support customers in making confident decisions.
Learn More

Featured Whitepapers and Reports
The State of Black Participation in Clinical Trials
In our new research report, we set forth to assess the current state of racial diversity beyond the FDA Snapshot, using Medidata’s industry-leading clinical trial data repository.

Featured Case Studies
Medidata Selected by Worldwide Clinical Trials to Accelerate Trials and Transform Patient Experience
Medidata and Worldwide Clinical Trials have expanded their partnership to leverage Medidata’s advanced solutions and extensive data set, enhancing Worldwide’s clinical operations with data-driven insights to accelerate trial timelines, improve site selection, and advance the life sciences industry.

Featured Podcasts and Webinars
ASCO Education: Racial Disparities in Clinical Trial Participation
Learn about the state of clinical trial diversity in clinical trials and what clinicians and trial sponsors can do to improve participant diversity.

Featured Blogs
3 Ways to Approach Challenges During Study Feasibility Processes
Real-time, industry data and predictive models enable pharmaceutical companies and CROs to improve clinical trial site selection, forecast enrollment timelines, and optimize study feasibility through accurate scenario analysis and site performance predictions.