
Accelerate Drug Discovery and Development
In silico software for drug discovery and development help streamline pharmaceutical R&D. Researchers can design and optimize drug candidates virtually before moving on to expensive lab testing, saving countless hours of research and thousands of dollars in R&D costs. Incorporation of these tools into existing workflows is crucial for modern drug discovery and development projects.
BIOVIA provides comprehensive software solutions that span the entire drug discovery and development process. Multidisciplinary R&D teams can collaborate easily to accelerate life-changing therapies to market, while leveraging AI, machine learning, and advanced modeling and simulation. These solutions are available as Software as a Service (SaaS) on the 3DEXPERIENCE® cloud platform, allowing easy access to high-performance cloud computing and regular updates with the most up-to-date scientific functionality.

End-to-End Small Molecule Therapeutics Design
BIOVIA’s collaborative solutions for small molecule therapeutics design help scientists identify high-quality drug candidates faster, at reduced costs. With solutions built over decades of expertise, BIOVIA offers state-of-the-art science powered by physics-based molecular modeling, AI and machine learning, and lab informatics in a unified cloud-based environment. Together these solutions support drug discovery teams from virtual target identification and lead optimization to real lab experimentation, providing a flexible and scalable approach to drug discovery. They improve decision making and collaboration across teams, while saving time, money, and other resources.

Market-Leading Biotherapeutics Design and Optimization
BIOVIA’s comprehensive modeling and simulation application, Discovery Studio Simulation, is a market-leading, cloud-native solution for biotherapeutics design and optimization. In addition to an extensive suite of advanced physics-based modeling methods, Discovery Studio Simulation allows easy access to cutting-edge AI and machine learning models, including AlphaFold2 and OpenFold for structure prediction, RFDiffusion for motif scaffolding and ProteinMPNN models for generating multiple new sequence variants. By combining these methods in a single environment, Discovery Studio Simulation supports complete workflows for in silico biotherapeutics discovery, from 3D modeling and structure prediction to protein-protein docking to humanization and to formulation optimization, reducing time to market and saving valuable resources in laborious biotherapeutics discovery research.
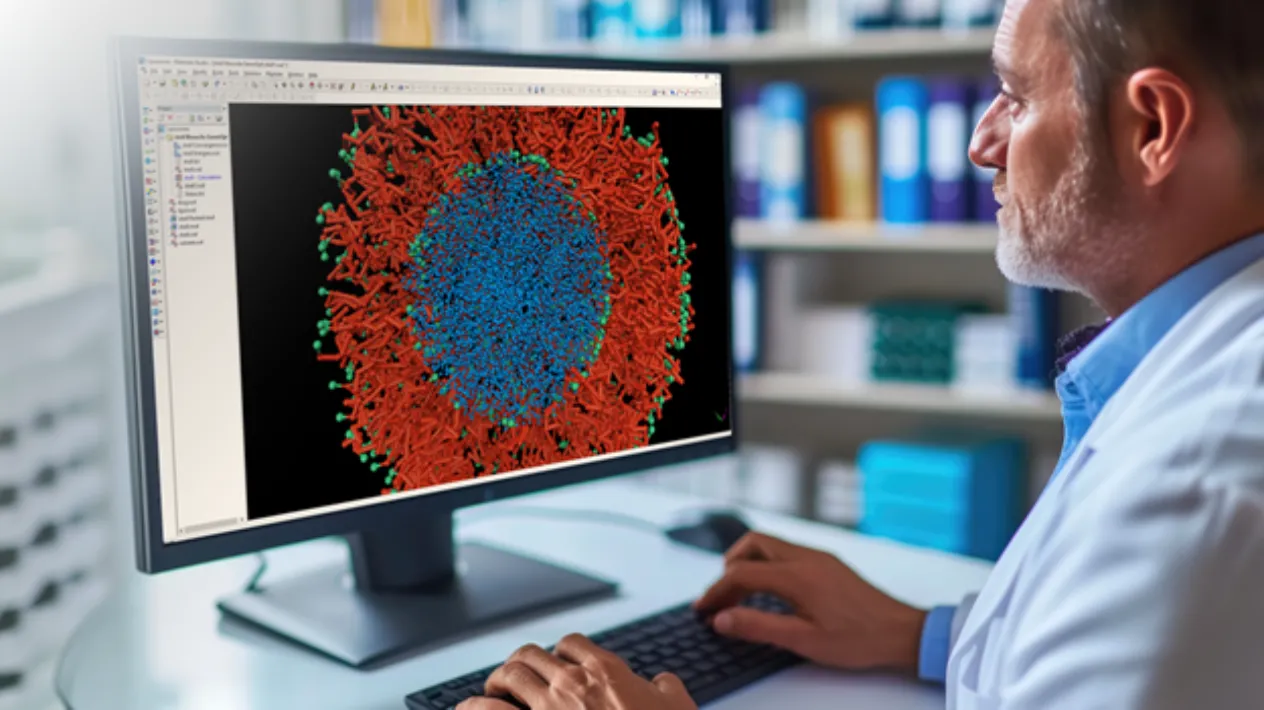
Advanced Pharmaceutical Development
BIOVIA provides drug development teams with advanced molecular modeling and simulation solutions, reducing costly trial-and-error experiments, supporting regulatory compliance, and enabling early risk assessment. With Materials Studio Simulation, users can predict API crystal structures and polymorphs; as a result, optimizing dosage form development and reducing late-stage failures. Users can also optimize formulation by evaluating solubility, stability, and mechanical properties of APIs and excipients. COSMO-RS helps further enhance solvent and co-crystal screening. Leading pharmaceutical companies use these BIOVIA solutions to cut costs, improve quality, and speed time to market.
Other Paths to Better Develop and Launch New Therapies
Discover More
Adopting an end-to-end solution for product development, manufacturing, and supply chain management will accelerate drug development.
Optimize resource utilization, reduce costs, improve collaboration and efficiency, and speed time-to-market.


